All Photos(2)
About This Item
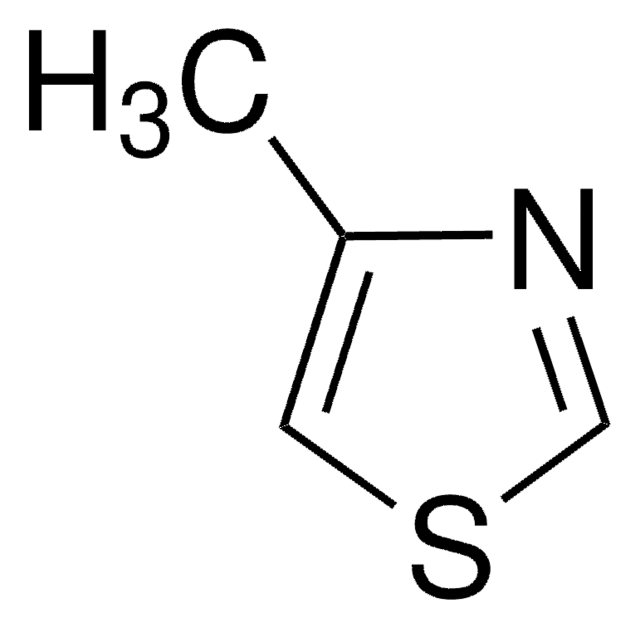
Empirical Formula (Hill Notation):
C4H5NS
CAS Number:
Molecular Weight:
99.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.524 (lit.)
bp
133-134 °C (lit.)
density
1.09 g/mL at 25 °C (lit.)
SMILES string
Cc1cscn1
InChI
1S/C4H5NS/c1-4-2-6-3-5-4/h2-3H,1H3
InChI key
QMHIMXFNBOYPND-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Methylthiazole forms complexes with cobalt(II), zinc(II), nickel(II) and copper(II) halides.
Application
4-Methylthiazole was used in the synthesis of catalytic dendrophanes, as functional mimics of the thiamine-diphosphate-dependent enzyme pyruvate oxidase. It was used in small scale preparation of 3-butyl-4-methylthiazolium bromide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
89.6 °F - closed cup
Flash Point(C)
32 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sarah E O'Connor et al.
Biochemistry, 41(17), 5685-5694 (2002-04-24)
The biosynthesis of epothilones, a family of hybrid polyketide (PK)/nonribosomal peptide (NRP) antitumor agents, provides an ideal system to study a hybrid PK/NRP natural product with significant biomedical value. Here the third enzyme involved in epothilone production, the five domain
Shivaji H Shelke et al.
Bioorganic & medicinal chemistry letters, 22(20), 6373-6376 (2012-09-18)
A new series of 3-aryl-2-(2-aryl/benzyl-4-methylthiazole-5-yl)thiazolidin-4-one was synthesized by condensation of 2-aryl/benzyl-4-methylthiazole-5-carbaldehyde, aromatic amines and thioglycolic acid in toluene. All the synthesized compounds are characterized by IR, NMR and elemental or mass analysis. Sixteen out of the newly synthesized compounds were
Metal complexes of 4-methylthiazole.
Hughes MN and Rutt KJ.
Inorganic Chemistry, 10(2), 414-416 (1971)
Improved preparations of ionic liquids using microwave irradiation.
Deetlefs M and Seddon KR.
Green Chemistry, 5(2), 181-186 (2003)
Catalytic Dendrophanes as Enzyme Mimics: Synthesis, Binding Properties, Micropolarity Effect, and Catalytic Activity of Dendritic Thiazolio-cyclophanes.
Habicher T, et al.
Helvetica Chimica Acta, 82(7), 1066-1095 (1999)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service