All Photos(3)
About This Item
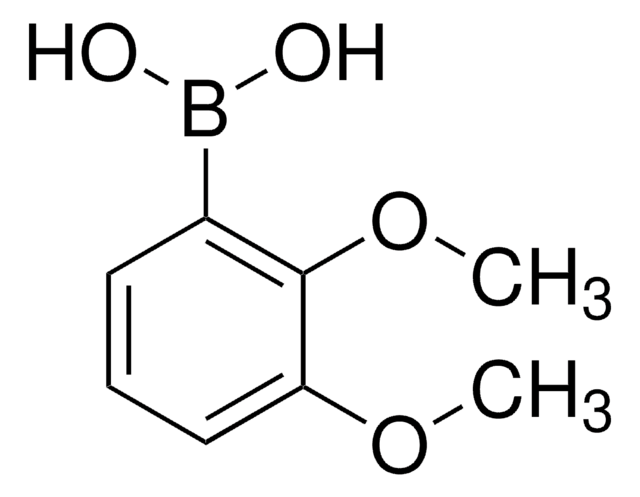
Linear Formula:
(CH3O)2C6H3B(OH)2
CAS Number:
Molecular Weight:
181.98
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
mp
245-250 °C (lit.)
SMILES string
COc1ccc(cc1OC)B(O)O
InChI
1S/C8H11BO4/c1-12-7-4-3-6(9(10)11)5-8(7)13-2/h3-5,10-11H,1-2H3
InChI key
RCVDPBFUMYUKPB-UHFFFAOYSA-N
Application
3,4-Dimethoxyphenylboronic acid can be used:
- As a substrate in the cross-coupling reaction with 5,7-dichloropyrido[4,3-d]pyrimidine catalyzed by palladium.
- As a starting material for the synthesis of buflavine 1, a natural alkaloid.
- In one of the key synthetic steps for the preparation of lipidated malarial glycosylphosphatidylinositols (GPI) disaccharide.
- To prepare 3,3″,4,4″-tetramethoxy-1,1′:4′,1″-terphenyl by reacting with 1,4-dibromobenzene using Pd catalyst.
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
3, 3″, 4, 4″-Tetramethoxy-1, 1′: 4′, 1″-terphenyl
Pui L, et al.
Acta Crystallographica Section E, Structure Reports Online, 67(Pt 8), o1892-o1892 (2011)
A Suzuki-Miyaura coupling mediated deprotection as key to the synthesis of a fully lipidated malarial GPI disaccharide
Liu X and Seeberger PH
Chemical Communications (Cambridge, England), 15, 1708-1709 (2004)
Highly efficient synthesis of buflavine: a unique Amaryllidaceae alkaloid
Sahakitpichan P and Ruchirawat S
Tetrahedron Letters, 44(28), 5239-5241 (2003)
Regioselective cross-coupling reactions and nucleophilic aromatic substitutions on a 5, 7-dichloropyrido [4, 3-d] pyrimidine scaffold
Jang M, et al.
Tetrahedron Letters, 47(50), 8917-8920 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)