All Photos(2)
About This Item
Linear Formula:
C6H5SO2CH2CN
CAS Number:
Molecular Weight:
181.21
Beilstein:
640716
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
112-114 °C (lit.)
functional group
nitrile
sulfone
SMILES string
O=S(=O)(CC#N)c1ccccc1
InChI
1S/C8H7NO2S/c9-6-7-12(10,11)8-4-2-1-3-5-8/h1-5H,7H2
InChI key
ZFCFFNGBCVAUDE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
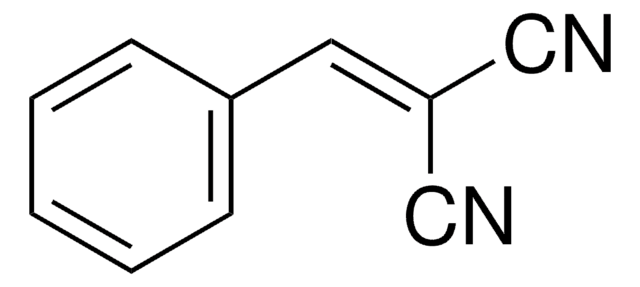
Condensation reaction of (phenylsulfonyl)acetonitrile with benzaldehyde in water in heterogeneous phase in the presence and absence of anionic and cationic surfactants has been studied. Dehydrative alkylation of alcohols with (phenylsulfonyl)acetonitrile under modified Mitsunobu conditions has been investigated.
Application
(Phenylsulfonyl)acetonitrile was used in the synthesis of pyridines, chromenes and thiophene derivatives based on sulfones.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Condensation reactions in water of active methylene compounds with arylaldehydes. One-pot synthesis of flavonols.
Fringuelli F, et al.
Tetrahedron, 50(39), 11499-11508 (1994)
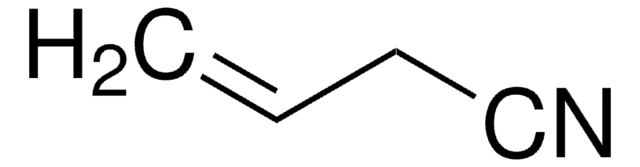
Two-carbon elongation/annulation of alcohols to nitriles.
Lai J-Y, et al.
Tetrahedron Letters, 36(32), 5691-5694 (1995)
Utility of sulphones in heterocyclic synthesis: Synthesis of some pyridine, chromene and thiophene derivatives.
Fadda AA, et al.
Macromolecules, 5(5), 701-709 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service