R0395
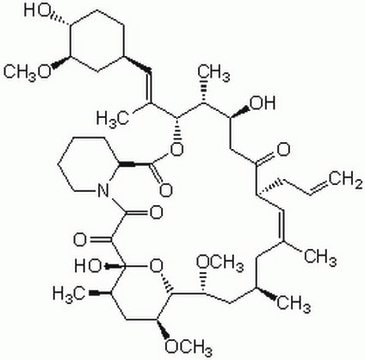
Rapamycin
from Streptomyces hygroscopicus, ≥95% (HPLC), powder, mTOR inhibitor
Synonym(s):
23,27-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine, AY 22989, Sirolimus
About This Item
Recommended Products
product name
Rapamycin from Streptomyces hygroscopicus, ≥95% (HPLC), powder
Quality Level
Assay
≥95% (HPLC)
form
powder
color
off-white
solubility
ethanol: 2 mM
DMSO: soluble
antibiotic activity spectrum
fungi
yeast
Mode of action
enzyme | inhibits
protein synthesis | interferes
storage temp.
−20°C
SMILES string
CO[C@@H]1C[C@@H](CC[C@H]1O)C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N4CCCC[C@H]4C(=O)O2)OC
InChI
1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1
InChI key
QFJCIRLUMZQUOT-HPLJOQBZSA-N
Gene Information
human ... FKBP1A(2280)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to treat BV2 cells or BV2-LC3 cells for autophagy assay
- as an autophagy activator in the bone marrow mesenchymal stem cells (BM-MSCs) transfected with lentivirus carrier of small interfering RNA for FOXO3 (siFOXO3) to study the effects of Forkhead Box O3 (FOXO3) on the autophagy of BM-MSCs
- to pre-incubate villi to inhibit the mammalian target of rapamycin complex 1 (mTORC1) signaling
Biochem/physiol Actions
Features and Benefits
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Cancer stem cell media, spheroid plates and cancer stem cell markers to culture and characterize CSC populations.
Cancer stem cell media, spheroid plates and cancer stem cell markers to culture and characterize CSC populations.
Cancer stem cell media, spheroid plates and cancer stem cell markers to culture and characterize CSC populations.
Cancer stem cell media, spheroid plates and cancer stem cell markers to culture and characterize CSC populations.
Related Content
Explore protein pathway analysis including chemical library screening and modulating pathways with small molecules.
Explore protein pathway analysis including chemical library screening and modulating pathways with small molecules.
Explore protein pathway analysis including chemical library screening and modulating pathways with small molecules.
Explore protein pathway analysis including chemical library screening and modulating pathways with small molecules.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service