81900
Propionitrile
purum, ≥99.0% (GC)
Synonym(s):
PPN, Ethyl cyanide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
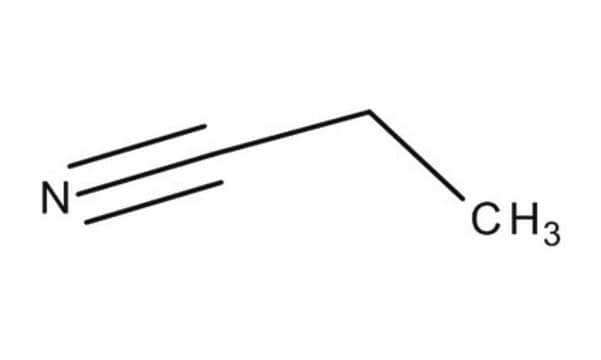
Linear Formula:
CH3CH2CN
CAS Number:
Molecular Weight:
55.08
Beilstein:
773680
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥99.0% (GC)
form
liquid
refractive index
n20/D 1.366 (lit.)
n20/D 1.366
bp
97 °C (lit.)
mp
−93 °C (lit.)
density
0.772 g/mL at 25 °C (lit.)
functional group
nitrile
SMILES string
CCC#N
InChI
1S/C3H5N/c1-2-3-4/h2H2,1H3
InChI key
FVSKHRXBFJPNKK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Propionitrile (PPN) is an effective solvent for catalytic asymmetric aldol reaction of a silyl enol ether with aldehydes in the presence of a chiral tin(II) Lewis acid catalyst.
- The co-solvent formed by mixing PPN with acetonitrile can be used to fabricate polymer gel electrolytes (PGEs) of dye-sensitized solar cells (DSSCs), which lead to enhanced stability of gel-state DSSCs.
- PPN can be used as a solvent for the Brønsted acid-catalyzed synthesis of N-alkyl cis-aziridines via [2+1] annulation of a diazo compound formed by the combination of an acetate and enolate. The process does not involve the use of metals or reagents and only atomic nitrogen as a co-product.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 2 Oral - Acute Tox. 4 Inhalation - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
42.8 °F - closed cup
Flash Point(C)
6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stability improvement of gel-state dye-sensitized solar cells by utilization the co-solvent effect of propionitrile/acetonitrile and 3-methoxypropionitrile/acetonitrile with poly (acrylonitrile-co-vinyl acetate).
Venkatesan S, et al.
Journal of Power Sources, 274, 506-511 (2015)
The Br?nsted acid-catalyzed direct Aza-Darzens synthesis of N-alkyl cis-aziridines.
Williams A L and Johnston J N
Journal of the American Chemical Society, 126(6), 1612-1613 (2004)
Catalytic asymmetric aldol-type reaction using a chiral tin (II) Lewis acid.
Kobayashi S, et al.
Tetrahedron, 49(9), 1761-1772 (1993)
D Somjen et al.
Journal of cellular biochemistry, 112(2), 625-632 (2011-01-27)
In cultured human osteoblasts estradiol-17β (E2) modulated DNA synthesis, the specific activity of creatine kinase BB (CK), 12 and 15 lipoxygenase (LO) mRNA expression and formation of 12- and 15-hydroxyeicosatetraenoic acid (HETE). We now investigate the response of human bone
H V O Carswell et al.
American journal of physiology. Heart and circulatory physiology, 287(4), H1501-H1504 (2004-05-25)
The present study employs selective estrogen receptor (ER) agonists to determine whether 17beta-estradiol-induced neuroprotection in global ischemia is receptor mediated and, if so, which subtype of receptor (ERalpha or ERbeta) is predominantly responsible. Halothane-anesthetized female C57Bl/6J mice were ovariectomized, and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service