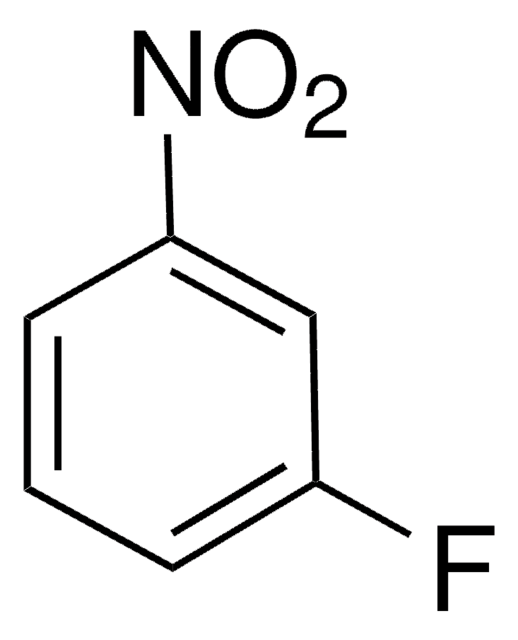
71210
Sodium ethoxide
technical, ≥95% (T)
Synonym(s):
Sodium ethylate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3CH2ONa
CAS Number:
Molecular Weight:
68.05
Beilstein:
3593646
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
vapor density
1.6 (vs air)
Quality Level
vapor pressure
<0.1 mmHg ( 20 °C)
grade
technical
Assay
≥95% (T)
form
powder
impurities
~2% Na2CO3 and NaOH
SMILES string
[Na+].CC[O-]
InChI
1S/C2H5O.Na/c1-2-3;/h2H2,1H3;/q-1;+1
InChI key
QDRKDTQENPPHOJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Sodium ethoxide (Sodium ethylate) is a sodium alkoxide. It has been synthesized by reacting sodium with ethanol. It undergoes decomposition in the presence of water to afford ethanol and sodium hydroxide. It is widely employed as a strong base in organic synthesis studies.
Sodium ethoxide is an alkoxide salt mainly used as a strong base in organic reactions such as deprotonation, dehydration and dehalogenation.
Application
Sodium ethoxide may be used as a base for the palladium catalyzed cross-coupling of aryl halides and alkenylboranes to synthesize arylated (E)-alkenes.
Sodium ethoxide may be used for the preparation of tricarbonylchloro(glycinato)ruthenium(II) (CORM-3).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Sol. 1 - Self-heat. 1 - Skin Corr. 1A
Supplementary Hazards
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 1
Flash Point(F)
86.0 °F - closed cup
Flash Point(C)
30 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst.
Miyaura N & Suzuki, A.
Journal of the Chemical Society. Chemical Communications, 19, 866-867 (1979)
James E Clark et al.
Circulation research, 93(2), e2-e8 (2003-07-05)
Carbon monoxide, which is generated in mammals during the degradation of heme by the enzyme heme oxygenase, is an important signaling mediator. Transition metal carbonyls have been recently shown to function as carbon monoxide-releasing molecules (CO-RMs) and to elicit distinct
Whitaker KS and Whitaker DT
e-EROS Encyclopedia of Reagents for Organic Synthesis (2001)
Eagleson M.
Concise Encyclopedia Chemistry, 997-997 (1994)
N C Luu et al.
Chemical research in toxicology, 13(7), 610-615 (2000-07-18)
Glutathione conjugate formation plays important roles in the detoxification and bioactivation of xenobiotics. A range of nephrotoxic haloalkenes undergo bioactivation that involves glutathione and cysteine S-conjugate formation. The cysteine S-conjugates thus formed may undergo cysteine conjugate beta-lyase-catalyzed biotransformation to form
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service