A86805
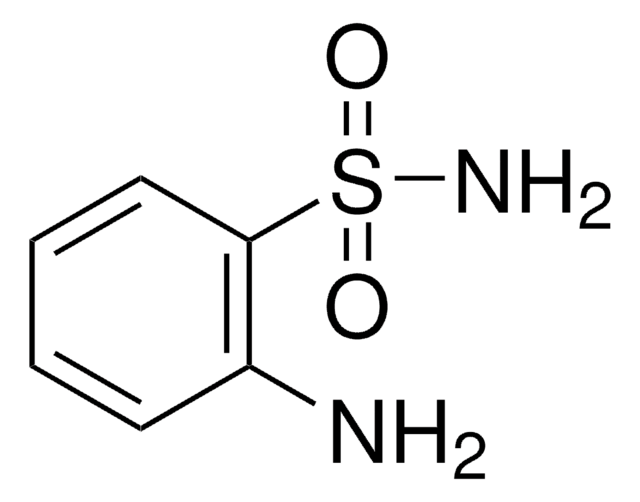
Aniline-2-sulfonic acid
95%
Synonym(s):
Orthanilic acid, 2-Aminobenzenesulfonic acid, o-Sulfanilic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2NC6H4SO3H
CAS Number:
Molecular Weight:
173.19
Beilstein:
1309204
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
SMILES string
Nc1ccccc1S(O)(=O)=O
InChI
1S/C6H7NO3S/c7-5-3-1-2-4-6(5)11(8,9)10/h1-4H,7H2,(H,8,9,10)
InChI key
ZMCHBSMFKQYNKA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J Mampel et al.
Microbiology (Reading, England), 145 ( Pt 11), 3255-3264 (1999-12-10)
Growth of Alcaligenes sp. strain O-1 with 2-aminobenzenesulfonate (ABS; orthanilate) as sole source of carbon and energy requires expression of the soluble, multicomponent 2-aminobenzenesulfonate 2,3-dioxygenase system (deaminating) (ABSDOS) which is plasmid-encoded. ABSDOS was separated by anion-exchange chromatography to yield a
F Junker et al.
The Biochemical journal, 300 ( Pt 2), 429-436 (1994-06-01)
2-Aminobenzenesulphonic acid (2AS) is degraded by Alcaligenes sp. strain O-1 via a previously detected but unidentified intermediate. A mutant of strain O-1 was found to excrete this intermediate, which was isolated and identified by m.s., 1H- and 13C-n.m.r. as 3-sulphocatechol
Inotropic activity of orthanilic and L-cysteic acid on isolated guinea-pig ventricular strips.
F Franconi et al.
Advances in experimental medicine and biology, 217, 159-165 (1987-01-01)
Xin-Gui Li et al.
Journal of combinatorial chemistry, 8(2), 174-183 (2006-03-15)
A unique strategy for synthesis of narrowly distributed and inherently self-stabilized copolymer nanoparticles by a simple emulsifier-free polymerization from orthanilic acid and aniline was developed. The polymerization yield, electrical conductivity, size, and its distribution of the nanoparticles could be simultaneously
Po-Hsin Wang et al.
Nanomaterials (Basel, Switzerland), 8(4) (2018-04-05)
In this work, we electrochemically deposited self-doped polyanilines (SPANI) on the surface of carbon-nanoparticle (CNP) film, enhancing the superficial faradic reactions in supercapacitors and thus improving their performance. SPANI was electrodeposited on the CNP-film employing electropolymerization of aniline (AN) and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service