665916
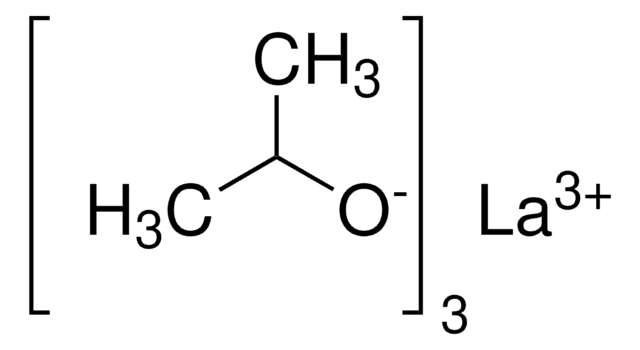
Yttrium(III) tris(isopropoxide)
Synonym(s):
2-Propanol yttrium(III) salt, Isopropyl alcohol yttrium(III) salt, Tris(isopropoxy) yttrium(III)
About This Item
Recommended Products
form
solid
reaction suitability
core: yttrium
reagent type: catalyst
SMILES string
CC(C)O[Y](OC(C)C)OC(C)C
InChI
1S/3C3H7O.Y/c3*1-3(2)4;/h3*3H,1-2H3;/q3*-1;+3
InChI key
PYLIDHFYDYRZSC-UHFFFAOYSA-N
Related Categories
Application
- Stereoselective conjugate addition reactions
- Ring-opening polymerizations
- Generation of reactive enolates by enantioselective protonation reactions
- Cyano-phosphorylation of enone and allylic substitution
- Synthesis of nanocomposites
Reactant for the preparation of flat HfO2 films
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Lanthanide ions in spectral conversion enhance solar cell efficiency via photon conversion.
Lanthanide ions in spectral conversion enhance solar cell efficiency via photon conversion.
Lanthanide ions in spectral conversion enhance solar cell efficiency via photon conversion.
Lanthanide ions in spectral conversion enhance solar cell efficiency via photon conversion.
Related Content
Professor Shibasaki's research focuses on the development of novel cooperative asymmetric catalytic systems that allowed for streamlined synthesis of enantioenriched high-value chiral building blocks.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![Tris[N,N-bis(trimethylsilyl)amide]yttrium](/deepweb/assets/sigmaaldrich/product/structures/867/983/5b7cb7cd-8879-49e4-a9d7-29c52aaa82a0/640/5b7cb7cd-8879-49e4-a9d7-29c52aaa82a0.png)