407518
Julolidine hydrobromide
97%
Synonym(s):
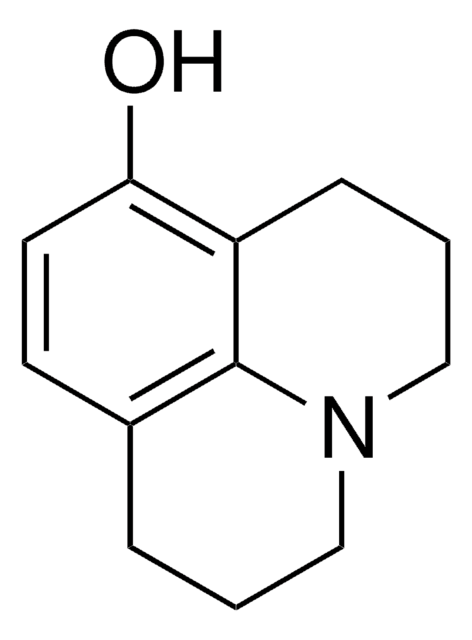
2,3,6,7-Tetrahydro-1H,5H-benzo[ij]quinolizine hydrobromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H15N · HBr
CAS Number:
Molecular Weight:
254.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
239-242 °C (lit.)
SMILES string
Br.C1CN2CCCc3cccc(C1)c23
InChI
1S/C12H15N.BrH/c1-4-10-6-2-8-13-9-3-7-11(5-1)12(10)13;/h1,4-5H,2-3,6-9H2;1H
InChI key
KHWBRFVBPGPEOJ-UHFFFAOYSA-N
General description
Julolidines are effective auxofluors that are employed in laser dyes and biochemical stains. They are known to be one of the strongest electron-releasing groups due to electronic and steric factors.
Application
Julolidine hydrobromide may be used in the synthesis of [4-(2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-9-ylazo)-phenyl]-methanol azodye.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of julolidine derivatives.
Kauffman JM, et al.
Organic preparations and procedures international, 33(6), 603-613 (2001)
Synthesis, optical characterization and crystal and molecular X-ray structure of a phenylazojulolidine derivative.
Barbero N, et al.
Dyes and Pigments, 92(3), 1177-1183 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service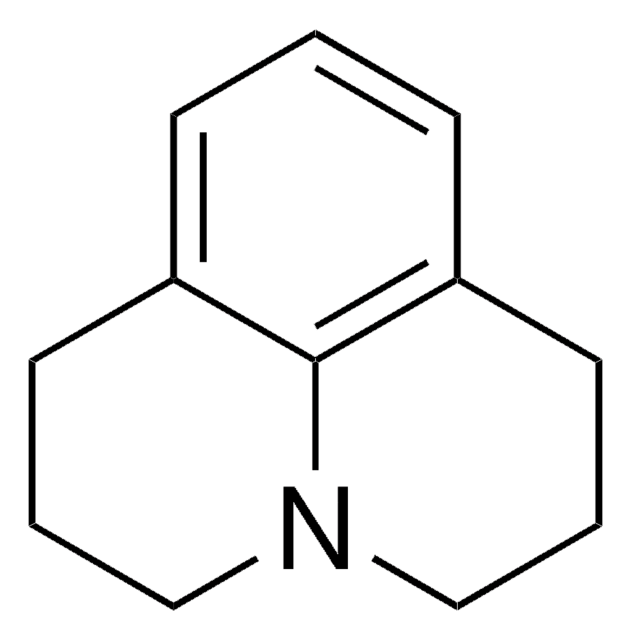
![2,3,6,7-Tetrahydro-8-hydroxy-1H,5H-benzo[ij]quinolizine-9-carboxaldehyde 98%](/deepweb/assets/sigmaaldrich/product/structures/166/830/a0d9a84a-5623-41a1-a54b-3b0272e5b28c/640/a0d9a84a-5623-41a1-a54b-3b0272e5b28c.png)