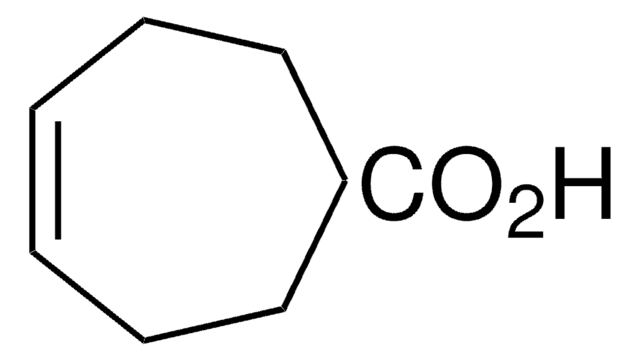
160687
2-Cyclohepten-1-one
80%, technical grade
Synonym(s):
2-Cycloheptenone, Tropilene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C7H10(=O)
CAS Number:
Molecular Weight:
110.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
80%
form
liquid
refractive index
n20/D 1.494 (lit.)
density
0.988 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
O=C1CCCCC=C1
InChI
1S/C7H10O/c8-7-5-3-1-2-4-6-7/h3,5H,1-2,4,6H2
InChI key
WZCRDVTWUYLPTR-UHFFFAOYSA-N
General description
2-Cyclohepten-1-one is an α,β-enone and its regioselective reaction with allyl indium reagent in the presence of TMSCl has been reported. It reacts with Bu2Zn in the presence of catalytic amounts of Cu(OTf)2 and CH2-bridged azolium salts to give (S)-3-butylcycloheptanone.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
154.4 °F - closed cup
Flash Point(C)
68 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T Masukawa et al.
Life sciences, 44(6), 417-424 (1989-01-01)
To search for a technique to deplete reduced glutathione (GSH) in brain, the influence of various types of compounds on brain GSH levels was investigated in mice. Of the compounds tested, cyclohexene-1-one, cycloheptene-1-one and diethyl maleate were shown to be
G Benzi et al.
Neurobiology of aging, 12(3), 227-231 (1991-05-01)
A severe age-dependent depletion of reduced glutathione (GSH) occurs in rat forebrain at 1-3 h from intraperitoneal injection of the electrophilic agents cyclohexene-1-one and cycloheptene-1-one. Chronic pretreatment with central dopamine agonists (i.e., ergot alkaloids; particularly, dihydroergocriptine) partially counteracts the GSH
Naoatsu Shibata et al.
The Journal of organic chemistry, 77(8), 4079-4086 (2012-03-30)
A series of hydroxy-amide functionalized azolium salts have been designed and synthesized for Cu-catalyzed asymmetric conjugate addition reaction. The (CH(2))(2)-bridged hydroxy-amide functionalized azolium ligand precursors 2, in addition to the previously reported CH(2)-bridged azolium salts 1, have been prepared from
P R Byron et al.
Journal of pharmaceutical sciences, 69(5), 527-531 (1980-05-01)
A stirred transfer cell containing equal volumes of light liquid paraffin and an aqueous phase at 37 degrees was used to demonstrate the feasibility of calculating the partition coefficient of an unstable compound by kinetic analysis. Cyclohept-2-enone was chosen since
Studies on the reactions of α, β-enones with allyl indium reagent; effects of TMSCl as promoter on regioselectivity.
Lee PH, et al.
Tetrahedron Letters, 42(1), 37-39 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service