153826
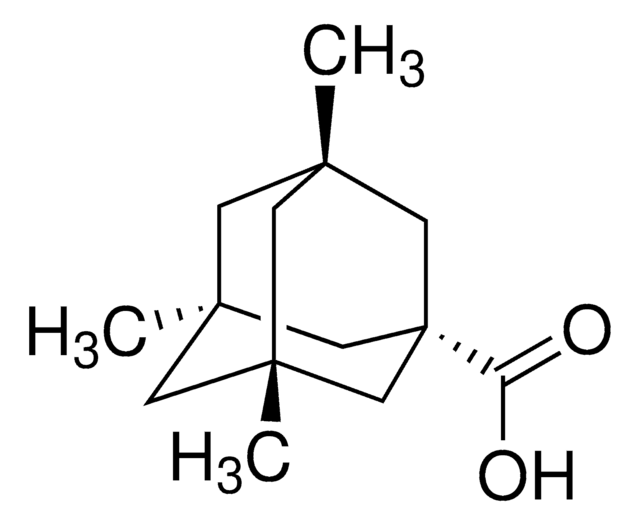
2-Adamantanol
97%
Synonym(s):
2-Hydroxyadamantane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H16O
CAS Number:
Molecular Weight:
152.23
Beilstein:
2498536
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
258-262 °C (lit.)
functional group
hydroxyl
SMILES string
OC1[C@H]2C[C@@H]3C[C@H](C2)C[C@H]1C3
InChI
1S/C10H16O/c11-10-8-2-6-1-7(4-8)5-9(10)3-6/h6-11H,1-5H2/t6-,7+,8-,9+,10?
InChI key
FOWDOWQYRZXQDP-MGPGSJOLSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The low-temperature X-ray structure of 2-adamantanol ester has been studied.
Application
2-Adamantanol was used to synthesize ester imides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T Mavromoustakos et al.
Life sciences, 62(20), 1901-1910 (1998-05-26)
Differential Scanning Calorimetry (DSC) has been applied to study the thermal properties of the membrane perturbing antibacterial octyl- and dodecyl-bromide salts of quaternary dimethylamino adamantanol (ADM-8 and ADM-12 correspondingly) incorporated in free or complexed form with beta-cyclodextrin (beta-CD) into dipalmitoylphosphatidylcholine
Andrzej Orzeszko et al.
Farmaco (Societa chimica italiana : 1989), 57(8), 619-624 (2002-10-04)
Some novel ester imides synthesised from trimellitic acid anhydride and 1-adamantanol or 2-adamantanol, were tested as antimicrobial compounds. Unfortunately, these agents showed a modest antibacterial activity (MIC > 6 microg/ml). However, a comparison of these N-substituted adamantylester imides with the
J P Miller et al.
Biochemistry, 33(3), 807-817 (1994-01-25)
Spiro[adamantane-2,2'-diazirine], which produces adamantyl carbene upon photolysis, binds tightly to P450 2B4 (KS = 3.2 microM), giving a normal substrate binding difference spectrum. Irradiation of 2-[3H]adamantane diazirine at 365 nm in the presence of native, ferric P450 2B4 resulted in
Koichi Mitsukura et al.
Applied microbiology and biotechnology, 71(4), 502-504 (2005-09-28)
Hydroxylation of adamantane using whole cells of bacteria, actinomyces, and molds was examined. The structure of the product was determined using gas chromatography (GC), nuclear magnetic resonance (NMR), and mass spectroscopy (MS). Among 470 strains tested, Streptomyces griseoplanus was highly
Marisa Spiniello et al.
Organic & biomolecular chemistry, 1(17), 3094-3101 (2003-10-02)
Results of low-temperature X-ray structural studies for five cis-, and three trans-4-tert-butyl cyclohexanol, and six 2-adamantanol ester and ether derivatives are reported. Plots of C-OR bond distance against pKa(ROH) for derivatives of axial alcohol (5), equatorial alcohol (6) and 2-adamantanol
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service