133485
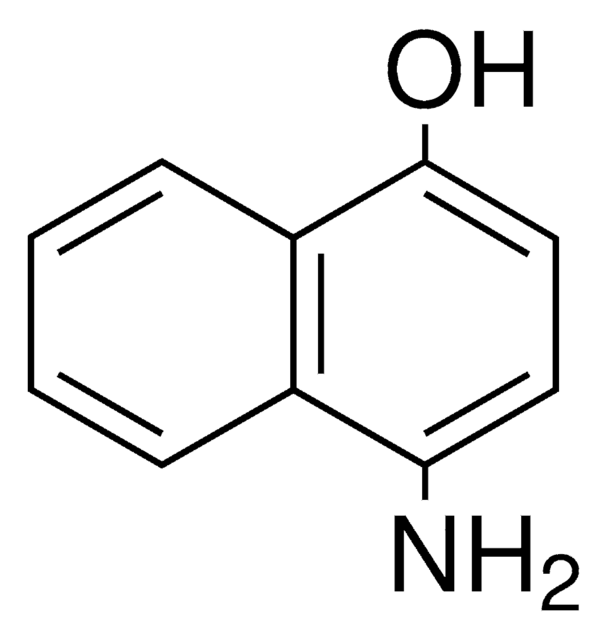
4-Amino-1-naphthol hydrochloride
technical grade, 90%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
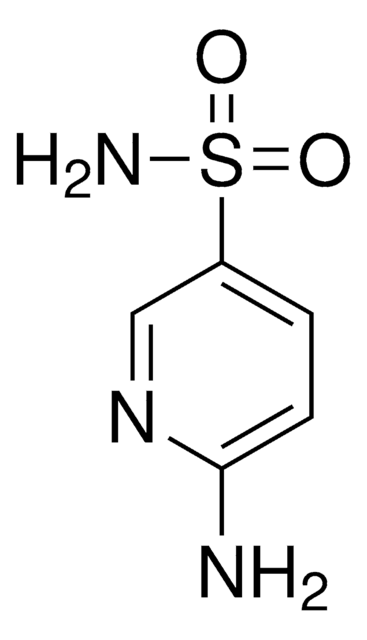
Linear Formula:
H2NC10H6OH · HCl
CAS Number:
Molecular Weight:
195.65
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
90%
form
solid
mp
273 °C (dec.) (lit.)
SMILES string
Cl.Nc1ccc(O)c2ccccc12
InChI
1S/C10H9NO.ClH/c11-9-5-6-10(12)8-4-2-1-3-7(8)9;/h1-6,12H,11H2;1H
InChI key
FDBQTRARWCKEJY-UHFFFAOYSA-N
Application
4-Amino-1-naphthol hydrochloride was used in the synthesis of 2-(3-aminophenol)-6-(4-amino-1-naphthol)-4-chloro-s-triazine. It was used in the synthesis of 4-aminoalkyl-1-naphthol derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S F Teng et al.
Journal of chromatography. B, Biomedical sciences and applications, 740(1), 1-15 (2000-05-08)
A synthetic bifunctional ligand (22/8) comprising a triazine scaffold substituted with 3-aminophenol (22) and 4-amino-1-naphthol (8) has been designed, synthesised, characterised and immobilized on agarose beads to create a robust, highly selective affinity adsorbent for human immunoglobulin G (IgG). Scatchard
Ute F Röhrig et al.
Journal of medicinal chemistry, 53(3), 1172-1189 (2010-01-09)
Indoleamine 2,3-dioxygenase (IDO) is an important therapeutic target for the treatment of diseases such as cancer that involve pathological immune escape. We have used the evolutionary docking algorithm EADock to design new inhibitors of this enzyme. First, we investigated the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service