123838
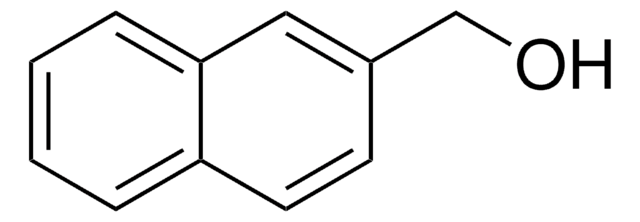
Biphenyl-4-methanol
98%
Synonym(s):
4-(Hydroxymethyl)biphenyl, 4-Phenylbenzyl alcohol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5C6H4CH2OH
CAS Number:
Molecular Weight:
184.23
Beilstein:
1937761
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
96-100 °C (lit.)
solubility
acetone: soluble 25 mg/mL
functional group
hydroxyl
phenyl
SMILES string
OCc1ccc(cc1)-c2ccccc2
InChI
1S/C13H12O/c14-10-11-6-8-13(9-7-11)12-4-2-1-3-5-12/h1-9,14H,10H2
InChI key
AXCHZLOJGKSWLV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Biphenyl-4-methanol acts as monofunctional alcohol initiator during the ring-opening polymerization of trimethylene carbonate (TMC) catalyzed by CH3SO3H and copolymerisation of ε-caprolactone and TMC.
Application
Biphenyl-4-methanol was used as reagent for the rapid quantitative determination of lithium alkyls.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A New Insight Into the Mechanism of the Ring-Opening Polymerization of Trimethylene Carbonate Catalyzed by Methanesulfonic Acid.
Campos JM, et al.
Macromolecular Chemistry and Physics, 214(1), 85-93 (2013)
The Journal of Organic Chemistry, 48, 2603-2603 (1983)
DNA damage and its repair in cultured human alveolar tumor cells treated with benzyl chloride, 4-chloromethylbiphenyl or 4-hydroxymethylbiphenyl.
R Mirzayans et al.
Mutation research, 100(1-4), 203-206 (1982-01-01)
UKEMS trial compounds: induction of unscheduled DNA synthesis in HeLa S3 cells.
R H Barrett
Mutation research, 100(1-4), 207-209 (1982-01-01)
A comparison of the response of BHK21 C13 cells to 4-chloromethylbiphenyl, 4-hydroxymethylbiphenyl and benzyl chloride in a cell transformation test.
J M Dehnel et al.
Mutation research, 100(1-4), 223-226 (1982-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service