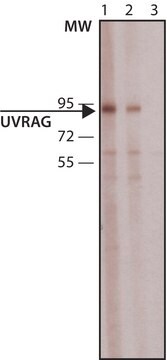
P8986
Peroxiredoxin 1 human
≥85% (SDS-PAGE), recombinant, expressed in E. coli, lyophilized powder
Synonym(s):
NKEFA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
human
Quality Level
recombinant
expressed in E. coli
Assay
≥85% (SDS-PAGE)
form
lyophilized powder
UniProt accession no.
application(s)
cell analysis
shipped in
wet ice
storage temp.
−20°C
Gene Information
human ... PRDX1(5052)
General description
Peroxiredoxin 1, an active enzyme, has two cysteine residues which correspond to Cys 47 and Cys 170 of yeast TPx (thioredoxin peroxidase).
Specificity
This product has been shown to catalyze NADPH oxidation in the thioredoxin-thioredoxin reductase system.
Application
Peroxiredoxin 1 has been used as a standard in enzyme assay for Clostridium difficile recombinant spore coat protein, cotE (with peroxiredoxin and chitinase activity).
Biochem/physiol Actions
Peroxiredoxin 1 has a role in cellular peroxide scavenging and it modulates various signaling pathways. It is over-expressed in many cancer cells. It regulates cell growth and signaling by interacting with proteins like c-jun-N-terminal kinase and c-myc.
Peroxiredoxins are a novel defined family of peroxidases of approximately 25 kDa that reduce H2O2 and alkyl hydroperoxides and use mainly the thioredoxin (Trx) system (Trx, thioredoxin reductase and NADPH) as electron donor. The peroxiredoxin family includes more than 30 proteins from organisms of all kingdoms. Peroxiredoxin I belongs to the Type I Peroxiredoxin family. This family consists of human natural killer cell enhancing factor (NKEFA), human proliferation associated gene (PAG), mouse macrophage stress induced protein (MSP23), mouse osteoblast specific factor (OSF-3), and rat heme-binding protein (HBP23). These proteins share about 95% homology.
Physical form
Lyophilized powder containing HEPES buffer salts and trehalose as stabilizer.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Functional characterization of Clostridium difficile spore coat proteins.
Permpoonpattana P
Journal of Bacteriology, 195(7, 1492-1503 (2013)
Chia-Hsiung Liu et al.
Journal of translational medicine, 14(1), 114-114 (2016-05-05)
Extracellular peroxiredoxin 1 (Prdx1) has been implicated to play a pivotal role in regulating inflammation; however, its function in tissue hypoxia-induced inflammation, such as severe cardiogenic shock patients, has not yet been defined. Thus, the objective of this study was
Overexpression of Prdx1 in hilar cholangiocarcinoma: a predictor for recurrence and prognosis.
Zhou J
International Journal of Clinical and Experimental Pathology, 8(9), 9863-9874 (2015)
H Z Chae et al.
The Journal of biological chemistry, 269(44), 27670-27678 (1994-11-04)
A 25-kDa antioxidant enzyme that provides protection against oxidation systems capable of generating reactive oxygen and sulfur species has previously been identified. The nature of the oxidant eliminated by, and the physiological source of reducing equivalents for, this enzyme, however
Pengfei Ren et al.
Oncology reports, 30(5), 2297-2303 (2013-09-07)
Peroxiredoxin 1 (Prdx1) is an antioxidant and plays an important role in H2O2-mediated cell signaling. We previously found that the expression level of Prdx1 was elevated in esophagus squamous cell carcinoma (ESCC) tissue using a proteomics approach. Since overexpressed protein
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service