M9005
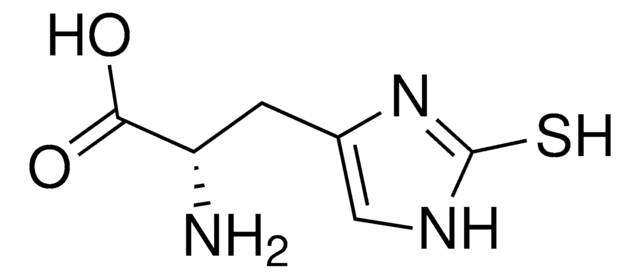
3-Methyl-L-histidine
≥98% (TLC)
Synonym(s):
π-Methyl-L-histidine, 1-Methyl-L-histidine (archaic), 3-(1-Methylimidazol-5-yl)-L-alanine
About This Item
Recommended Products
product name
3-Methyl-L-histidine,
Assay
≥98% (TLC)
form
powder
color
white to off-white
mp
254 °C
application(s)
detection
SMILES string
Cn1cncc1C[C@H](N)C(O)=O
InChI
1S/C7H11N3O2/c1-10-4-9-3-5(10)2-6(8)7(11)12/h3-4,6H,2,8H2,1H3,(H,11,12)/t6-/m0/s1
InChI key
JDHILDINMRGULE-LURJTMIESA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Engineering mutually orthogonal PylRS/tRNA pairs for dual encoding of functional histidine analogues.: Exploring innovative genetic engineering techniques, this research advances the use of histidine analogues like 3-Methyl-L-histidine for producing novel proteins with enhanced functionalities, offering vast potential in therapeutic and industrial applications (Taylor et al., 2023).
- Improving the enzymatic activity of l-amino acid α-ligase for imidazole dipeptide production by site-directed mutagenesis.: This investigation enhances the enzymatic synthesis of imidazole dipeptides, crucial for understanding the role of modifications like those seen in 3-Methyl-L-histidine and its impact on biological activities (Kino et al., 2023).
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service