All Photos(2)
About This Item
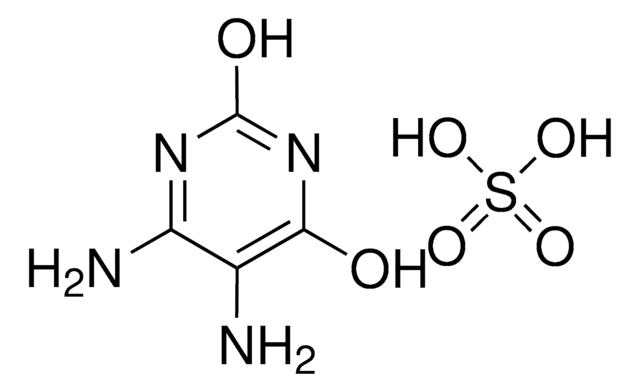
Empirical Formula (Hill Notation):
C10H12N2O4
CAS Number:
Molecular Weight:
224.21
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
Assay
≥97% (TLC)
Quality Level
form
powder
solubility
methanol: 50 mg/mL, clear, colorless to faintly yellow
storage temp.
−20°C
SMILES string
CC1=C[N@@H]2[C@H]3C[C@@H](OC2=NC1=O)[C@@H](CO)O3
InChI
1S/C10H12N2O4/c1-5-3-12-8-2-6(7(4-13)15-8)16-10(12)11-9(5)14/h3,6-8,13H,2,4H2,1H3/t6-,7-,8-/m1/s1
InChI key
JCSNHEYOIASGKU-BWZBUEFSSA-N
Application
2,3′-Anhydrothymidine may be derivitized to 5′-benzoyl-2′,3′-anhydrothymidine for use in the synthesis of 5′-Derivatives of 3′-(tetrazole-2′-yl)-3′-deoxythymidines. 2,3′-Anhydrothymidine may be contacted with thioacetic acid to produce 3′-S-acetyl-3′-thio-2′-deoxynucleosides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
V A Ostrovskiĭ et al.
Bioorganicheskaia khimiia, 21(1), 49-54 (1995-01-01)
5''-Derivatives of 3'-(tetrazole-2''-yl)-3'-deoxythymidines were synthesized by interaction of 5'-benzoyl-2',3'-anhydrothymidine with tetrazole or its 5-derivatives followed by debenzoylation. Structures of two of them, 3'-(tetrazole-2''-yl)-3'-deoxythymidine and 3'-(5''-methyltetrazole-2''-yl)-3'-deoxythymidine, elucidated by X-ray analysis, revealed anti conformation of thymine about the glycosidic bond and 2'-endo-3'-exo-conformation
Peihua Shang et al.
Nucleosides, nucleotides & nucleic acids, 27(12), 1272-1281 (2008-11-13)
A general method is described for synthesizing 3',5'-dithio-2'-deoxypyrimidine nucleosides 6 and 13 from normal 2'-deoxynucleosides. 2,3'-Anhydronucleosides 2 and 9 are applied as intermediates in the process to reverse the conformation of 3'-position on sugar rings. The intramolecular rings of 2,3'-anhydrothymidine
Maren Schulze et al.
Methods in molecular biology (Clifton, N.J.), 329, 45-58 (2006-07-19)
The in vitro differentiation of mouse embryonic stem (ES) cells into different somatic cell types such as neurons, endothelial cells, or myocytes is well established, and many mouse ES cell lines have been created so far. The establishment of rat
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service