P0532
Phthaldialdehyde Reagent
Solution Complete
Synonym(s):
OPA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
storage temp.
2-8°C
SMILES string
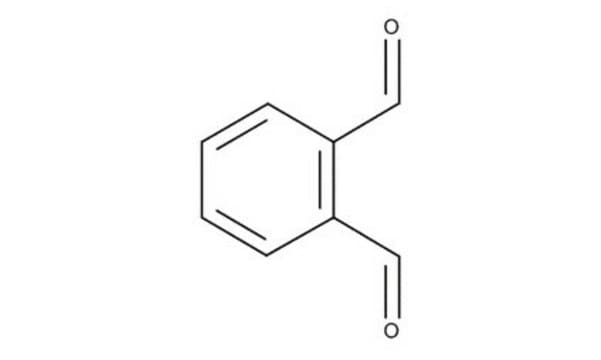
[H]C(=O)c1ccccc1C([H])=O
InChI
1S/C8H6O2/c9-5-7-3-1-2-4-8(7)6-10/h1-6H
InChI key
ZWLUXSQADUDCSB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Precolumn derivatization reagent for primary amines and amino acids. The fluorescent derivative can be detected by reverse-phase HPLC. The reaction requires OPA, primary amine and a sulfhydryl. In the presence of excess sulfhydryl, amines can be quantitated. In the presence of excess amine, sulfhydryls can be quantitated.
Other Notes
Contains 1 mg o-phthaldialdehyde (P0657) per mL solution with 2-mercaptoethanol as the sulfhydryl moiety.
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Dam. 1 - Met. Corr. 1 - Repr. 1B - Skin Corr. 1B - Skin Sens. 1
WGK
WGK 3
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Silke B Lohan et al.
Antioxidants (Basel, Switzerland), 9(6) (2020-06-25)
The daily consumption of tobacco products leads to a boost in free radical production in tissues, promoting the risk for malignancies, metabolic alterations and chronic-inflammatory diseases. This study aimed to broaden the knowledge of the status of the antioxidative (AO)
Katsuhiro Suzuki et al.
Biomedical chromatography : BMC, 27(4), 535-538 (2012-09-29)
Monomethylarginine, asymmetric dimethylarginine and symmetric dimethylarginine were separated on a Wakopak Combi ODS with an acetonitrile-100 mm potassium phosphate buffer (pH 7.0; 1:1, v/v). Dimethylarginines were derived from o-phthalaldehyde for the fluorescence detector and from 6-ferrocenyl-1-hexanethiol for the electrochemical detector. The
Noriko Nishino et al.
Environmental science & technology, 46(15), 8198-8204 (2012-07-20)
Naphthalene, typically the most abundant polycyclic aromatic hydrocarbon in the atmosphere, reacts with OH radicals by addition to form OH-naphthalene adducts. These OH-naphthalene adducts react with O(2) and NO(2), with the two reactions being of equal importance in air at
Quansong Li et al.
Physical chemistry chemical physics : PCCP, 14(18), 6561-6568 (2012-03-30)
The potential energy surface for the intramolecular excited state hydrogen transfer (IESHT) in ortho-phthalaldehyde (OPA), which generates an enol ketene, has been studied with ab initio calculations (MS-CASPT2//CASSCF). The goal of our study is to establish the mechanistic factors that
Vishnu Menon et al.
Journal of fluorescence, 23(2), 311-321 (2012-12-06)
This is the first report of inactivation of xyloglucanase from Thermomonospora sp by pepstatin A, a specific inhibitor towards aspartic proteases. The steady state kinetics revealed a reversible, competitive, two-step inhibition mechanism with IC 50 and K i values of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service