91737
Trifluoromethanesulfonic anhydride
purum, ≥98.0% (T)
Synonym(s):
Tf2O, Triflic anhydride
About This Item
Recommended Products
vapor density
5.2 (vs air)
Quality Level
vapor pressure
8 mmHg ( 20 °C)
grade
purum
Assay
≥98.0% (T)
form
liquid
refractive index
n20/D 1.321 (lit.)
bp
81-83 °C (lit.)
density
1.677 g/mL at 25 °C (lit.)
SMILES string
FC(F)(F)S(=O)(=O)OS(=O)(=O)C(F)(F)F
InChI
1S/C2F6O5S2/c3-1(4,5)14(9,10)13-15(11,12)2(6,7)8
InChI key
WJKHJLXJJJATHN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reagent for stereoselective synthesis of mannosazide methyl uronate donors
Activator for direct glycosylation with anomeric hydroxy sugars
Tf2O in combination with:
- Diphenyl sulfoxide, forms an efficient promoter system for the activation of thioglycosides for glycosylation reactions.
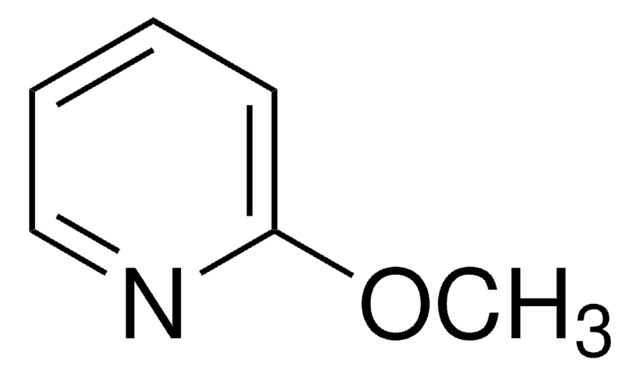
- 2-chloropyridine, can be used for secondary amide activation.
- Potassium iodide, can effectively mediate the deoxygenation of sulfoxides to form sulfides.
- Hydrogen peroxide, for the selective oxidation of sulfanes to sulfoxides in the presence of oxidatively sensitive functional groups.
Tf2O can react with:
- Ketones in the presence of a base to form vinyl triflates.
- Magnesium alkoxides to form corresponding symmetrical or unsymmetrical ethers.
- Sodium azide for the in situ preparation of triflyl azide.
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Ox. Liq. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
not determinedboils before flash
Flash Point(C)
not determinedboils before flash
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service






![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)