36574
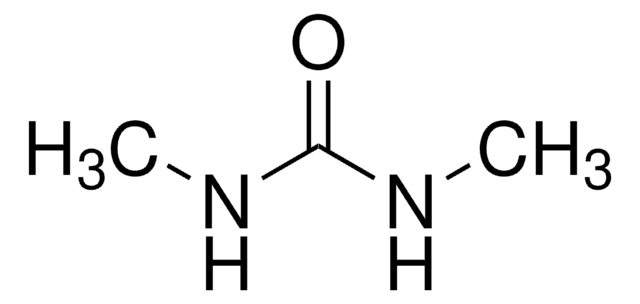
N,N′-Dimethylurea
PESTANAL®, analytical standard
Synonym(s):
1,3-Dimethylurea
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3NH)2CO
CAS Number:
Molecular Weight:
88.11
Beilstein:
1740672
EC Number:
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
description
(sym.)
product line
PESTANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
bp
268-270 °C (lit.)
mp
101-104 °C (lit.)
application(s)
agriculture
environmental
format
neat
SMILES string
CNC(=O)NC
InChI
1S/C3H8N2O/c1-4-3(6)5-2/h1-2H3,(H2,4,5,6)
InChI key
MGJKQDOBUOMPEZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Legal Information
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
314.6 °F - closed cup
Flash Point(C)
157 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yishan Chen et al.
Physical chemistry chemical physics : PCCP, 13(16), 7384-7395 (2011-03-23)
The binding behaviors of the 27-membered macrocyclic triurea 1 towards the five anions, F(-), Cl(-), Br(-), I(-) and NO(3)(-), through multiple hydrogen-bonding interactions, were investigated at the B3LYP/6-311++G(d,p)//B3LYP/6-31(1)++G(d,p) (6-31(1)++G(d,p) is a hybrid basis set; for more details see computational methods)
Dimethylurea: a radical scavenger that protects isolated pancreatic islets from the effects of alloxan and dihydroxyfumarate exposure.
L J Fischer et al.
Life sciences, 26(17), 1405-1409 (1980-04-28)
Anna Ronowicz et al.
Analytical biochemistry, 426(2), 91-93 (2012-04-17)
Reuse of materials in DNA hybridization-based methods has been known since the advent of Southern membranes. Array-based comparative genomic hybridization is essentially Southern hybridization with multiple probes immobilized on a solid surface. We show that comparative genomic hybridization microarrays fabricated
M M Santoro et al.
Biochemistry, 27(21), 8063-8068 (1988-10-18)
Characteristics and properties of the unfolding free energy change, delta G degrees N-U, as determined by the linear extrapolation method are assessed for the unfolding of phenylmethanesulfonyl chymotrypsin (PMS-Ct). Difference spectral measurements at 293 nm were used to define PMS-Ct
In vitro stimulation by paraquat of reactive oxygen-mediated lipid peroxidation in rat lung microsomes.
M A Trush et al.
Toxicology and applied pharmacology, 60(2), 279-286 (1981-09-15)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service