10815
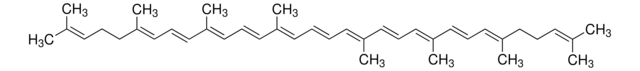
Ethyl β-apo-8′-carotenoate (trans)
≥80% (TLC)
Synonym(s):
Ethyl 8′-apo-β,ψ-caroten-8′-oate, all-trans-Carophyll yellow
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C32H44O2
CAS Number:
Molecular Weight:
460.69
Beilstein:
2021845
EC Number:
MDL number:
UNSPSC Code:
12352200
Recommended Products
Assay
≥80% (TLC)
impurities
≤0.001% heavy metals
ign. residue
≤0.1%
loss
≤0.1% loss on drying
mp
134-136 °C
solubility
chloroform: 10 mg/mL at 25 °C, clear, intense red-orange
storage temp.
−20°C
SMILES string
CCOC(=O)\C(C)=C\C=C\C(C)=C\C=C\C=C(C)\C=C\C=C(C)\C=C\C1=C(C)CCCC1(C)C
Other Notes
Internal standard in: Individual carotenoid determinations in human plasma by HPLC
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A A Franke et al.
Journal of chromatography, 614(1), 43-57 (1993-04-21)
A method is described for the synthesis of new carotenoids by transesterification of ethyl-beta-apo-8'-carotenoate. The rate of transesterification was temperature-dependent with highest yields using primary alcohols. Reaction conditions were found avoiding Z isomerization. The structures of the new products were
C.C. Tangney
Journal of Liquid Chromatography, 7, 2611-2611 (1984)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service