902845
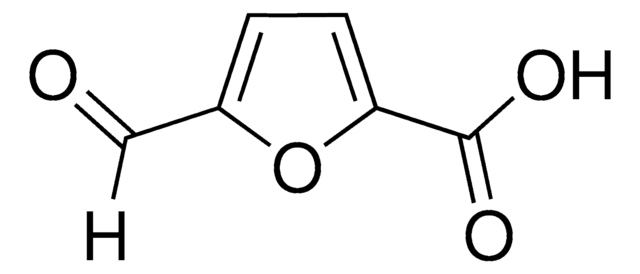
5-Hydroxymethyl-2-furancarboxylic acid
≥95%
Synonym(s):
5-(Hydroxymethyl)-2-furoic Acid, NSC 40739, Sumikis′ Acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H6O4
CAS Number:
Molecular Weight:
142.11
MDL number:
UNSPSC Code:
12352005
NACRES:
NA.22
Recommended Products
Assay
≥95%
form
powder or crystals
storage temp.
−20°C
InChI
1S/C6H6O4/c7-3-4-1-2-5(10-4)6(8)9/h1-2,7H,3H2,(H,8,9)
InChI key
PCSKKIUURRTAEM-UHFFFAOYSA-N
General description
5-Hydroxymethyl-2-furancarboxylic acid (HMFCA), a versatile building block in the polymer industry, is commonly used in polyester production and servs as an essential precursor to synthesize renewable terephthalic acid. Also, used as a monomer in the production of polyethylene terephthalate (PET).
Application
- Photocatalytic applications in renewable energy: Research highlights the role of Zn(x)Cd(1-x)S nanoparticles, where 5-Hydroxymethyl-2-furancarboxylic acid is used to enhance photocatalytic water splitting for hydrogen production. This process supports sustainable energy solutions (Pham et al., 2023).
- Biodegradable polymer synthesis: A study on the synthesis of biodegradable oligoesters from ε-caprolactone and 5-Hydroxymethyl-2-furancarboxylic acid using immobilized lipases demonstrates its potential in creating environmentally friendly materials (Todea et al., 2019).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Selective synthesis of 2-furoic acid and 5-hydroxymethyl-2-furancarboxylic acid from bio-based furans by recombinant Escherichia coli cells
Shi SS,? et al.
Molecular Catalysis, 469, 68-74 (2019)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service