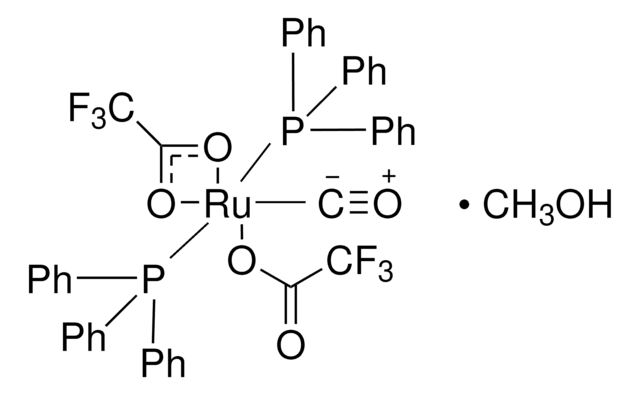
901235
TBS-DHG Catalyst
≥95%
Synonym(s):
(2R,3S,4R)-2-(((tert-Butyldimethylsilyl)oxy)methyl)tetrahydro-2H-pyran-3,4-diol, 6-Tertbutyldimethylsilyl-1,2-dihydroglucal
About This Item
Recommended Products
Quality Level
Assay
≥95%
form
powder or crystals
reaction suitability
reagent type: catalyst
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
greener alternative category
, Aligned
storage temp.
−20°C
SMILES string
[H]C1([H])C([H])([H])O[C@@](C([H])([H])O[Si](C([H])([H])[H])(C([H])([H])[H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])([H])[C@](O[H])([H])[C@]1([H])O[H]
Related Categories
General description
Application
Other Notes
- Carbohydrate-Catalyzed Enantioselective Alkene Diboration:Enhanced Reactivity of 1,2-Bonded Diboron Complexes
- Diols, α-Ketols, and Diones as 22π Components in [2+2+2] Cycloadditions of 1,6-Diynes via Ruthenium(0)-Catalyzed Transfer Hydrogenation
- Carbohydrate/DBU Cocatalyzed Alkene Diboration: Mechanistic Insight Provides Enhanced Catalytic Efficiency and Substrate Scope
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
Chiral organoboronic esters are well known as versatile intermediates for chemical synthesis. Not only are these compounds stable under a variety of reaction conditions, they are generally non-toxic and can be transformed with minimal generation of hazardous waste.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)

![Dichloro[9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene]palladium(II) 95%](/deepweb/assets/sigmaaldrich/product/structures/374/597/f7932c5b-0448-498b-8254-f8ce1b9a4612/640/f7932c5b-0448-498b-8254-f8ce1b9a4612.png)