764566
Cobalt sulfide
99.98% trace metals basis
Synonym(s):
Cobalt disulfide, Cobalt(IV) sulfide
About This Item
Recommended Products
Assay
99.98% trace metals basis
form
powder
mol wt
Mw 123.06 g/mol
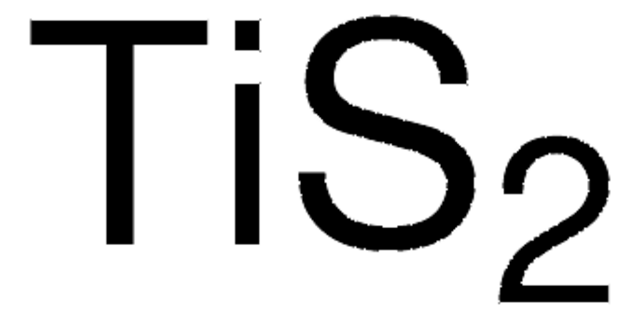
composition
CoS2
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
average diameter
≤10 μm
particle size
≤10 μm
density
4.23 g/cm3 (lit.)
application(s)
battery manufacturing
greener alternative category
SMILES string
S=[Co]=S
InChI
1S/Co.2S
InChI key
XUKVMZJGMBEQDE-UHFFFAOYSA-N
Related Categories
General description
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Solid oxide fuel cells and electrolyzers show potential for chemical-to-electrical energy conversion, despite early development stages.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service