712256
5-Azidopentanoic acid
≥97.0%
Synonym(s):
5-Azidovalerianic acid
About This Item
Recommended Products
Assay
≥97.0% (TLC)
≥97.0%
97.0-103.0% (calc. on dry substance, T)
form
liquid
availability
available only in USA
loss
≤10% loss on drying
storage temp.
−20°C
SMILES string
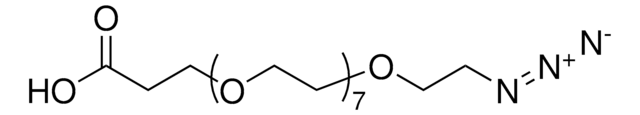
OC(=O)CCCCN=[N+]=[N-]
InChI
1S/C5H9N3O2/c6-8-7-4-2-1-3-5(9)10/h1-4H2,(H,9,10)
InChI key
SBZDIRMBQJDCLB-UHFFFAOYSA-N
Application
- Chitosan-poly(ethylene glycol) hydrogels by azide–alkyne click chemistry.
- 5-iodo-1,2,3-triazole containing macrocycles.
- Bivalent quinine dimers intervening triazole ring used as inhibitors of P-glycoprotein (P-gp) mediated cellular efflux.
Packaging
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 1B - Self-react. C
Supplementary Hazards
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service











![2-[2-(2-Azidoethoxy)ethoxy]ethanol solution ~0.5 M in tert-butyl methyl ether](/deepweb/assets/sigmaaldrich/product/structures/374/007/eea7ca74-41e4-4aac-af71-c93c37ec0a5a/640/eea7ca74-41e4-4aac-af71-c93c37ec0a5a.png)