592862
3-Chloro-benzo[b]thiophene-2-carboxylic acid
97%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H5ClO2S
CAS Number:
Molecular Weight:
212.65
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
268-272 °C (lit.)
SMILES string
OC(=O)c1sc2ccccc2c1Cl
InChI
1S/C9H5ClO2S/c10-7-5-3-1-2-4-6(5)13-8(7)9(11)12/h1-4H,(H,11,12)
InChI key
HJTMIYKPPPYDRJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Chloro-benzo[b]thiophene-2-carboxylic acid may be used in the synthesis of the following:
- 5-(3-chlorobenzo[b]thiophen-2-yl)-1,3,4-thiadiazol-2-ylamine via condensation with thiosemicarbazide in the presence of POCl3
- 2-benzo[b]thiophen-2-yl-6-methylbenzo[d][1;3]oxazi-4-one via reaction with 2-amino-5-methyl-benzoic acid in the presence of carbonyl diimidazole
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and biological activity of new 1,3,4-thiadiazole derivatives?
Aly.A.A and Sayed-LE.R
Chemical Papers, 60(01), 56-60 (2006)
Neutral Inhibitors of the Serine Protease Factor Xa?
Shrader.DW, et al.
Bioorganic & Medicinal Chemistry Letters, 11(14), 1801- 1804 (2001)
Michael D Neinast et al.
Cell metabolism, 29(2), 417-429 (2018-11-20)
Elevations in branched-chain amino acids (BCAAs) associate with numerous systemic diseases, including cancer, diabetes, and heart failure. However, an integrated understanding of whole-body BCAA metabolism remains lacking. Here, we employ in vivo isotopic tracing to systemically quantify BCAA oxidation in healthy
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service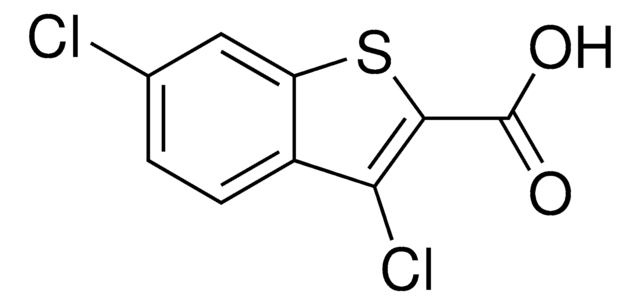

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)






