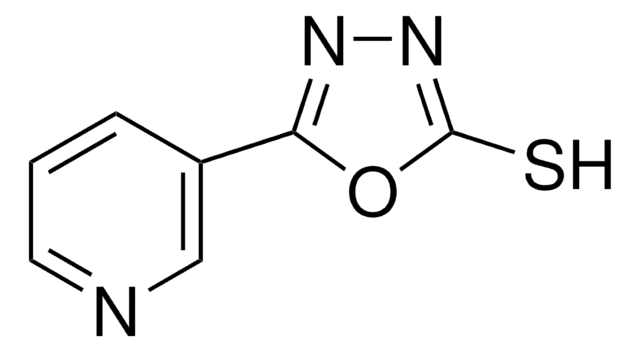
55087
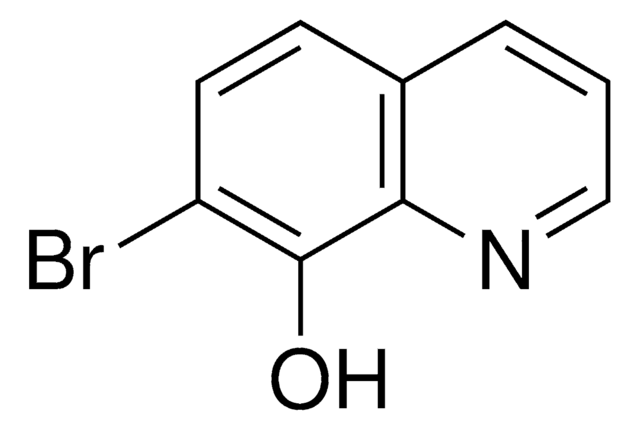
8-Hydroxy-2-quinolinecarbonitrile
≥98.0% (GC)
Synonym(s):
8-Hydroxyquinaldonitrile
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H6N2O
CAS Number:
Molecular Weight:
170.17
Beilstein:
473250
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (GC)
SMILES string
Oc1cccc2ccc(nc12)C#N
InChI
1S/C10H6N2O/c11-6-8-5-4-7-2-1-3-9(13)10(7)12-8/h1-5,13H
InChI key
KUQKKIBQVSFDHX-UHFFFAOYSA-N
Application
8-Hydroxy-2-quinolinecarbonitrile may be used in the preparation of 8-hydroxy-2-quinolinemethylamine, a reagent to generate peptoid oligomers.
Other Notes
Building block for preparing chelating agents
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C. Moberg et al.
Journal of the Chemical Society. Chemical Communications, 810-810 (1988)
Heterocyclic amines for the construction of peptoid oligomers bearing multi-dentate ligands.
Maayan G, et al.
Tetrahedron Letters, 49(2), 335-338 (2008)
Vladimir Majerciak et al.
Journal of virology, 93(2) (2018-10-26)
Epstein-Barr virus (EBV) is a ubiquitous human pathogen associated with Burkitt's lymphoma and nasopharyngeal carcinoma. Although the EBV genome harbors more than a hundred genes, a full transcription map with EBV polyadenylation profiles remains unknown. To elucidate the 3' ends
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service