All Photos(1)
About This Item
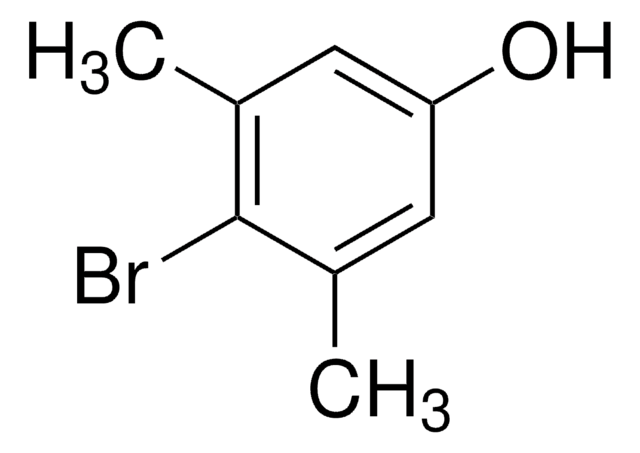
Linear Formula:
Br(CH3)2C6H2OCH3
CAS Number:
Molecular Weight:
215.09
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
96%
refractive index
n20/D 1.566 (lit.)
bp
197 °C (lit.)
density
1.36 g/mL at 25 °C (lit.)
SMILES string
COc1ccc(Br)c(C)c1C
InChI
1S/C9H11BrO/c1-6-7(2)9(11-3)5-4-8(6)10/h4-5H,1-3H3
InChI key
WOWNZZYJCUUIFC-UHFFFAOYSA-N
General description
4-Bromo-2,3-dimethylanisole can be prepared from 2,3-dimethylanisole via bromination using N-bromosuccinimide-acetonitrile (NBS-CH3CN) system.
Application
4-Bromo-2,3-dimethylanisole may be used in the preparation of:
- 4,6-Dibromo-2,3-dimethylanisole via bromination using NBS-CH3CN system.
- 4-Methoxy-2,2′,3,3′-tetramethyldiphenyl ether by reacting with 2,3-dimethylphenol.
- A novel arylsulfonamide derivative with potent glucocorticoid receptor (GR) agonist activity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Substituted phenyl as a steroid A-ring mimetic: Providing agonist activity to a class of arylsulfonamide nonsteroidal glucocorticoid ligands.
Darren D, et al.
Bioorganic & Medicinal Chemistry Letters, 23(24), 6645-6649 (2013)
Oxidative coupling of phenols. Part 9. The role of steric effects in the oxidation of methyl-substituted phenols.
Armstrong DR, et al.
J. Chem. Soc. Perkin Trans. II, 5, 581-585 (1983)
N-Bromosuccinimide in acetonitrile: A mild and regiospecific nuclear brominating reagent for methoxybenzenes and naphthalenes.
Carreno MC, et al.
The Journal of Organic Chemistry, 60(16), 5328-5331 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service