493937
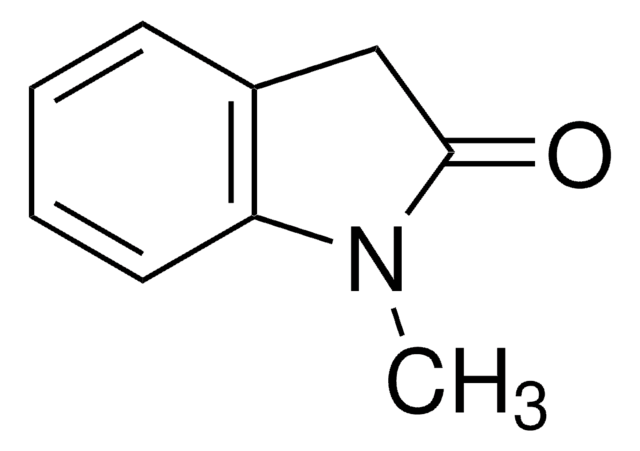
3-Methyl-2-oxindole
96%
Synonym(s):
3-Methyloxindole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H9NO
CAS Number:
Molecular Weight:
147.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
mp
117-121 °C (lit.)
SMILES string
CC1C(=O)Nc2ccccc12
InChI
1S/C9H9NO/c1-6-7-4-2-3-5-8(7)10-9(6)11/h2-6H,1H3,(H,10,11)
InChI key
BBZCPUCZKLTAJQ-UHFFFAOYSA-N
Related Categories
General description
3-Methyl-2-oxindole (MOI) is a 3-substituted-2-oxindole. It is reported to be formed during the oxidation of indole-3-acetic acid in the presence of FeII under aerobic conditions. MOI undergoes asymmetric anti-Mannich-type reaction with N-tosyl aryl aldimines in the presence of alkaloid cinchona derivatives to form anti-3,3-disubsituted 2-oxindole derivatives. It also undergoes asymmetric hydroxyamination with nitrosoarenes to form N-nitroso aldol products.
Application
3-Methyl-2-oxindole may be used in the preparation of 3-hydroxy-3-methyl-2-oxindole.
- Reactant for enantioselective α-amination reactions
- Reactant for aldol reaction with glyoxal derivatives
- Reactant for amine thiourea catalyzed conjugate addition to α,β-unsaturated aldehydes
- Reactant for O-acetylation reactions
- Reactant for preparation of a disubstituted oxoindole by using rhodium-catalyzed cyclopropanation/ring-opening reactions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Facile and Efficient Enantioselective Hydroxyamination Reaction: Synthesis of 3-Hydroxyamino-2-Oxindoles Using Nitrosoarenes.
Shen K, et al.
Angewandte Chemie (Weinheim an der Bergstrasse, Germany), 123(20), 4780-4784 (2011)
Jaroslav Matal et al.
Neuro endocrinology letters, 30 Suppl 1, 36-40 (2009-12-23)
To study the contribution of individual purified porcine CYP1A2, 2E1 and 2A19 enzymes to the biotransformation of skatole. Individual porcine and human enzymes (CYP1A2, 2E1 or 2A6/19) were used to study their potential involvement in skatole metabolism. Furthermore, the inhibition
Ying Jin et al.
Chirality, 26(12), 801-805 (2014-07-22)
A series of cinchona alkaloid derivatives were used to catalyze the asymmetric anti-Mannich-type reaction of 3-methyl-2-oxindole with N-tosyl aryl aldimines. The resulting anti-3,3-disubstituted 2-oxindole products were obtained in good yields (up to 92%) with high diastereo- and enantioselectivities (anti/syn up
Metabolism and pneumotoxicity of 3-methyloxindole, indole-3-carbinol, and 3-methylindole in goats.
M J Potchoiba et al.
American journal of veterinary research, 43(8), 1418-1423 (1982-08-01)
Takuma Sakurai et al.
Microorganisms, 7(9) (2019-09-14)
Recent studies have shown that metabolites produced by microbes can be considered as mediators of host-microbial interactions. In this study, we examined the production of tryptophan metabolites by Bifidobacterium strains found in the gastrointestinal tracts of humans and other animals.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service