342300
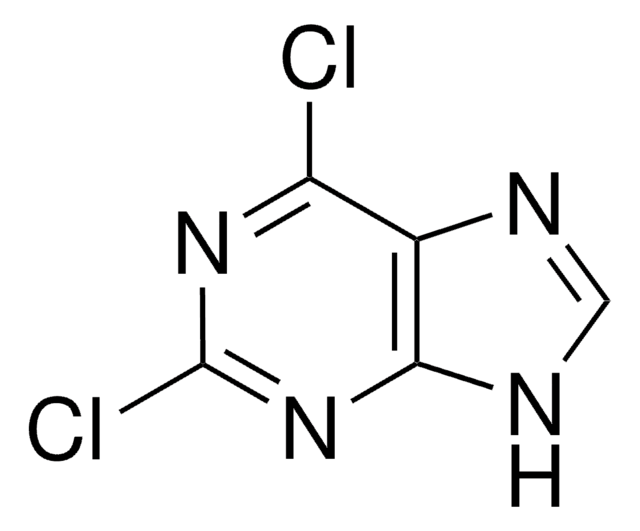
2-Amino-6-chloropurine
97%
Synonym(s):
6-Chloro-2-purinamine, 6-Chloroguanine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H4ClN5
CAS Number:
Molecular Weight:
169.57
Beilstein:
9626
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
>300 °C (lit.)
SMILES string
Nc1nc(Cl)c2nc[nH]c2n1
InChI
1S/C5H4ClN5/c6-3-2-4(9-1-8-2)11-5(7)10-3/h1H,(H3,7,8,9,10,11)
InChI key
RYYIULNRIVUMTQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Amino-6-chloropurine is a 6-substituted purine. Tautomeric purine forms of 2-amino-6-chloropurine were investigated by vibrational spectroscopy and quantum chemical method.
Application
2-Amino-6-chloropurine may be used:
- in the enzymatic synthesis of 2′-deoxyguanosine
- in the synthesis of 9-alkyl purines
- in the synthesis of (R)- and (S)-N-(2-phosphonomethoxypropyl) derivatives of purine and pyrimidine bases
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of enantiomeric N-(2-phosphonomethoxypropyl) derivatives of purine and pyrimidine bases. II. The synthon approach.
Holy A, et al.
Collection of Czechoslovak Chemical Communications, 60(8), 1309-1409 (1995)
K Kim et al.
Combinatorial chemistry & high throughput screening, 3(2), 125-129 (2000-05-02)
A new application of solid-supported reagents was developed to separate the alkylated N7/N9 regioisomers derived from commercially available 2-amino-6-chloropurine. Simple filtration through an alumina/H+ pad or scavenging by AG/Dowex-50W-X8 resin provides diverse N9 regioisomers selectively in moderate yields with high
V Balachandran et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 96, 340-351 (2012-06-19)
Two purine tautomers of 2-amino-6-chloropurine (ACP), in labeled as N(9)H(10) and N(7)H(10), were investigated by vibrational spectroscopy and quantum chemical method. The FT-IR and FT-Raman spectra of ACP have been recorded in the regions 4000-400 cm(-1) and 3500-100 cm(-1), respectively.
L L Bennett et al.
Biochemical pharmacology, 33(2), 261-271 (1984-01-15)
2-Amino-6-chloro-1-deazapurine is of interest as a purine analog with demonstrated in vivo activity against mouse leukemia L1210. That the active form of this agent is a nucleotide and that the nucleotide is formed by the action of hypoxanthine (guanine) phosphoribosyltransferase
Jan Novák et al.
Organic letters, 5(5), 637-639 (2003-02-28)
Protecting the hydroxyl group in both 2-bromo-2-phenylethanol and 2-bromo-1-phenylethanol enhanced the alkylation of 2-amino-6-chloropurine to give corresponding 7- and 9-alkylated products. Subsequent hydrolysis and deprotection led to 7- and 9-hydroxy(phenyl)ethylguanines. 7-Hydroxy(phenyl)ethylguanines are major guanine adducts formed by interaction of styrene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service