All Photos(2)
About This Item
Empirical Formula (Hill Notation):
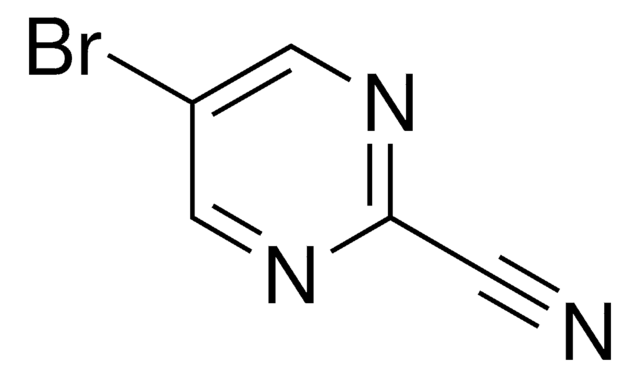
C4H3BrN2
CAS Number:
Molecular Weight:
158.98
Beilstein:
107326
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
67-73 °C (lit.)
SMILES string
Brc1cncnc1
InChI
1S/C4H3BrN2/c5-4-1-6-3-7-2-4/h1-3H
InChI key
GYCPLYCTMDTEPU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Rapid nucleophilic displacement reactions of 5-bromopyrimidine with nucleophiles under microwave irradiation has been studied. 5-Bromopyrimidine undergoes direct metallation with lithuium diisopropylamide to yield 4-lithio-5-bromopyrimidine.
Application
5-Bromopyrimidine was used in the synthesis of:
- N-heteroaryl substituted 9-arylcarbazolyl derivatives via palladium-catalyzed aerobic and ligand-free Suzuki reaction
- (5-(phenylethynyl)pyrimidine) via microwave assisted organic synthesis (MAOS) Sonogashira protocol
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Efficient nucleophilic substitution reactions of pyrimidyl and pyrazyl halides with nucleophiles under focused microwave irradiation.
Cherng,Y-J.
Tetrahedron, 58(5), 887-890 (2002)
Direct metalation of pyrimidine. Synthesis of some 4-substituted pyrimidines.
Kress TJ.
The Journal of Organic Chemistry, 44(13), 2081-2082 (1979)
The new NHS: restricting GPs' access to x ray services.
J Bahrami et al.
BMJ (Clinical research ed.), 302(6791), 1541-1541 (1991-06-22)
Xiaofeng Rao et al.
Organic & biomolecular chemistry, 10(39), 7875-7883 (2012-08-15)
A palladium-catalyzed aerobic and ligand-free Suzuki reaction in aqueous ethanol has been developed for the synthesis of N-heteroaryl substituted 9-arylcarbazolyl derivatives. A number of N-heteroaryl halides, namely 2-halogenated pyridines, 2-bromoquinoline, 5-bromopyrimidine and 2-chloropyrazine, were coupled with 4-(9H-carbazol-9-yl)phenylboronic acid (CPBA) or
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service