I601
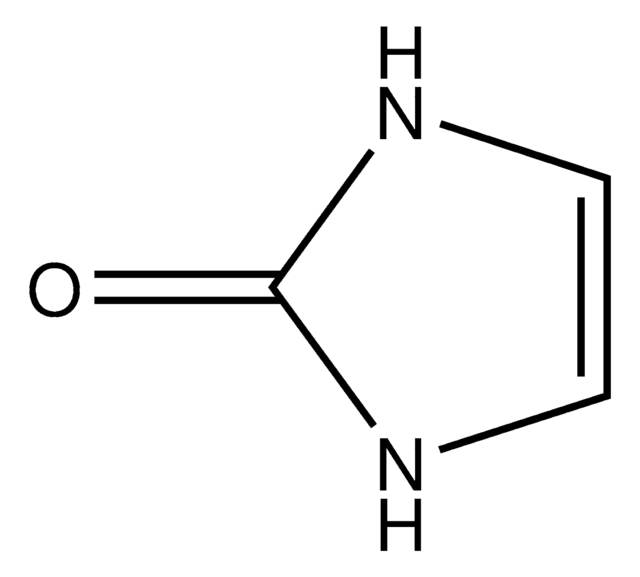
2-Imidazolidone
96%
Synonym(s):
2-Oxoimidazolidine, N,N′-Ethyleneurea
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H6N2O
CAS Number:
Molecular Weight:
86.09
Beilstein:
106252
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
mp
129-132 °C (lit.)
SMILES string
O=C1NCCN1
InChI
1S/C3H6N2O/c6-3-4-1-2-5-3/h1-2H2,(H2,4,5,6)
InChI key
YAMHXTCMCPHKLN-UHFFFAOYSA-N
Gene Information
human ... EPHX2(2053)
mouse ... Ephx2(13850)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Reactant for synthesis of:
Reactant for:
- Chiral microporous materials from achiral precursors
- Aryl and heteroaryl N-acylureas via microwave-assisted palladium-catalyzed carbonylation
- A highly water-soluble peptide based human neutrophil elastase inhibitor
- Heterocycles by cyanoacetylation for antimicrobial use
Reactant for:
- Pd-catalyzed C-N bond formation with heteroaromatic tosylates
- Oxidative amidation of activated alkenes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - STOT RE 2
Target Organs
Thyroid
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yasunao Inoue et al.
Bioorganic & medicinal chemistry, 17(21), 7477-7486 (2009-10-09)
A series of peptide-based transition-state human neutrophil elastase (HNE) inhibitors with N-terminal acidic moieties were synthesized and their inhibitory activity against HNE was evaluated both in vitro and in vivo. Our results show that compounds containing cyclic amide bridged acidic
New Synthesis of Aryl and Heteroaryl N-Acylureas via Microwave-Assisted Palladium-Catalyzed Carbonylation
D. Liptrot, et al.,
Advanced Synthesis & Catalysis, 352, 2183-2188 (2010)
Mette L H Mantel et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(18), 5437-5442 (2010-04-09)
A protocol for the palladium(0)-catalyzed amidation of heteroaromatic tosylates was successfully developed. The methodology proved to be effective for a variety of heteroaryl tosylates including the pyridine, pyrimidine, quinoline and quinoxaline ring systems. Successful carbon-nitrogen bond formation with these heteroaryl
D A Kuznetsov et al.
Cancer letters, 34(1), 61-66 (1987-01-01)
Daily intragastric administration of a carcinogen N,N'-ethylenethiourea (ETU) (85 mg/kg body wt, congruent to 0.1 DL50) leads to significant polyadenylate polymerase (PAP) activity in rat blood serum by the 10th day of experiment. A similar course of N,N'-ethyleneurea (EU) fails
Shelley L Aldrich et al.
Military medicine, 176(5), 584-585 (2011-06-04)
Ethylene urea/melamine formaldehyde resin (permanent press) is a common fabric finishing agent added to Army Combat Uniforms for a wrinkle-free appearance and to strengthen the fabric. We describe the case of an active duty U.S. Army soldier with a diffuse
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service