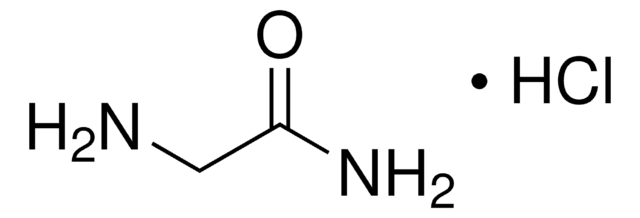
G6104
Glycinamide hydrochloride
98%
Synonym(s):
2-Aminoacetamide hydrochloride, Aminoacetamide hydrochloride, Glycine amide hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
NH2CH2CONH2 · HCl
CAS Number:
Molecular Weight:
110.54
Beilstein:
3554199
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
204 °C (dec.) (lit.)
SMILES string
Cl.NCC(N)=O
InChI
1S/C2H6N2O.ClH/c3-1-2(4)5;/h1,3H2,(H2,4,5);1H
InChI key
WKNMKGVLOWGGOU-UHFFFAOYSA-N
Application
Buffer useful in the physiological pH range.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Irene M Lagoja et al.
Chemistry & biodiversity, 2(7), 923-927 (2006-12-29)
A possible reaction mechanism for the dehydration of glycinamide (3) and N,N'-diformylurea (4) yielding hypoxanthine (2) has been investigated. Furthermore, a potential prebiotic route converting hypoxanthine (2) into adenine (1) via phosphate activation followed by substitution reaction with NH3 was
Stefan Glatzel et al.
Chemical communications (Cambridge, England), 46(25), 4517-4519 (2010-05-21)
Homopolymers of N-acryloyl glycinamide were prepared by reversible addition-fragmentation chain transfer polymerization in water. The formed macromolecules exhibit strong polymer-polymer interactions in aqueous milieu and therefore form thermoreversible physical hydrogels in pure water, physiological buffer or cell medium.
Irene M Lagoja et al.
Chemistry & biodiversity, 1(1), 106-111 (2006-12-29)
Because of their easy availability and their relative chemical stability, urea, formic acid, and glycine might have played a role in the assembly process of nucleobases. In this paper, a short reaction path is described to prepare hypoxanthine starting from
Yong Sun et al.
The journal of physical chemistry. B, 109(12), 5919-5926 (2006-07-21)
For the purpose of investigating the tautomerism from glycinamide (G) to glycinamidic acid (G*) induced by proton transfer, we carried out a study of structural interconversion of the two tautomers and the relative stabilizing influences of water during the tautomerization
Len Ito et al.
FEBS letters, 585(3), 555-560 (2011-01-18)
Glycine amide (GlyAd), a typically amidated amino acid, is a versatile additive that suppresses protein aggregation during refolding, heat treatment, and crystallization. In spite of its effectiveness, the exact mechanism by which GlyAd suppresses protein aggregation remains to be elucidated.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service