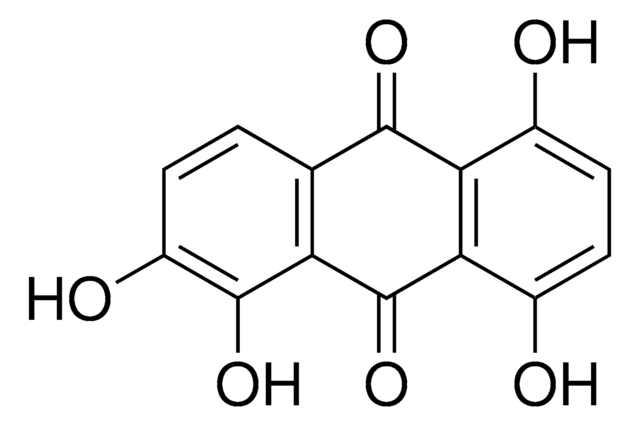
A89502
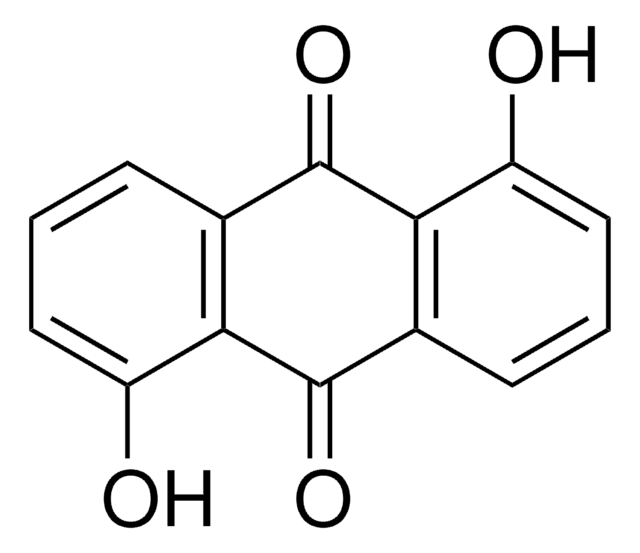
Anthraflavic acid
technical grade, 90%
Synonym(s):
2,6-Dihydroxyanthraquinone, Anthraflavine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C14H8O4
CAS Number:
Molecular Weight:
240.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
90%
form
powder
mp
>320 °C (lit.)
SMILES string
Oc1ccc2C(=O)c3cc(O)ccc3C(=O)c2c1
InChI
1S/C14H8O4/c15-7-1-3-9-11(5-7)14(18)10-4-2-8(16)6-12(10)13(9)17/h1-6,15-16H
InChI key
APAJFZPFBHMFQR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Anthraflavic acid can be used as a starting material to synthesize tetrahydroxy tetrathiafulvalene (TTF) derivatives, which are used as redox-active building blocks in supramolecular and materials science. It is also utilized to prepare phosphanylidene anthra[2,1-b]furans by reacting with dialkyl acetylenedicarboxylates and triphenylphosphine.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Regioselective synthesis of novel functionalized phosphanylidene anthra [2, 1-b] furan derivatives under solvent-free conditions
Nourmohammadian F and Gholami MD
Phosphorus, Sulfur, and Silicon and the Related Elements, 185(2), 340-346 (2010)
A D Ayrton et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 26(11-12), 909-915 (1988-11-01)
Administration of the antimutagen anthraflavic acid to rats gave rise to significant increases in the hepatic microsomal O-deethylations of ethoxyresorufin and ethoxycoumarin, but not in the O-dealkylation of pentoxyresorufin nor in cytosolic glutathione S-transferase activity. Immunoblot studies of solubilized microsomes
A D Ayrton et al.
Biochimica et biophysica acta, 916(3), 328-331 (1987-12-18)
Consideration of the computer-optimised dimensions of anthraflavic acid indicates that it is essentially a planar molecule with a large area/depth ratio, that would preferentially interact with the polycyclic aromatic hydrocarbon-induced family of cytochrome P-450 proteins (cytochromes P-448). Anthraflavic acid was
Synthesis of tetrahydroxy-?-extended tetrathiafulvalenes as new supramolecular redox building blocks
Diaz Marta C, et al.
Tetrahedron Letters, 44(5), 945-948 (2003)
A D Ayrton et al.
Mutation research, 207(3-4), 121-125 (1988-03-01)
The ability of anthraflavic acid to inhibit the mutagenicity of IQ was investigated using the Ames test and employing hepatic activation systems from Aroclor 1254-pretreated rats. Incorporation of anthraflavic acid into the S9 mix caused a concentration-dependent decrease in the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service