All Photos(2)
About This Item
Empirical Formula (Hill Notation):
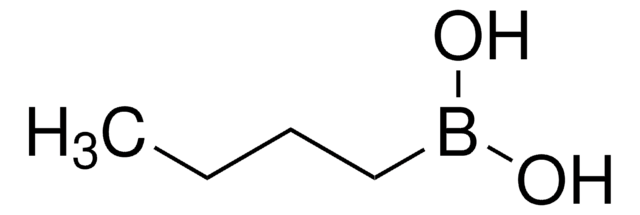
C4H9BO2
CAS Number:
Molecular Weight:
99.92
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0%
form
solid
mp
118-123 °C (lit.)
storage temp.
2-8°C
SMILES string
OB(O)C1CCC1
InChI
1S/C4H9BO2/c6-5(7)4-2-1-3-4/h4,6-7H,1-3H2
InChI key
MIUALDDWOKMYDA-UHFFFAOYSA-N
Related Categories
Application
- Reagent used in palladium-catalyzed arylation and alkylation of diphenylisoxazole with boronic acids via C-H activated isoxazole palladacycle intermediate
- Reagent used in the preparation of cyclobutyl arenes and heteroarenes via palladium catalyzed Suzuki-Miyaura cross-coupling reaction of potassium cyclobutyltrifluoroborate intermediate with aryl and heteroaryl chlorides
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Palladium-Catalyzed Arylation and Alkylation of 3,5-Diphenylisoxazole with Boronic Acids via C-H Activation
J. Chu, et al.,
Organometallics, 27, 5173-5176 (2008)
Gary A Molander et al.
The Journal of organic chemistry, 73(19), 7481-7485 (2008-09-02)
Suitable conditions were found for the Suzuki-Miyaura cross-coupling reaction of potassium cyclopropyl- and cyclobutyltrifluoroborates with aryl chlorides. Both of these secondary alkyl trifluoroborates coupled in moderate to excellent yield with electron-rich, electron-poor, and hindered aryl chlorides to give a variety
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service