425117
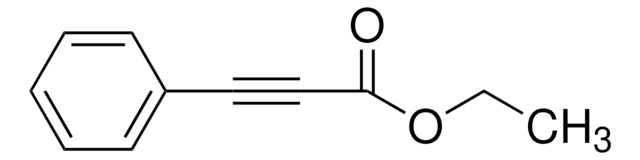
Ethyl 2-butynoate
98%
Synonym(s):
Ethyl tetrolate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C≡CC(O)OCH2CH3
CAS Number:
Molecular Weight:
112.13
Beilstein:
1744948
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.436 (lit.)
bp
160-161 °C/730 mmHg (lit.)
density
0.962 g/mL at 25 °C (lit.)
functional group
ester
SMILES string
CCOC(=O)C#CC
InChI
1S/C6H8O2/c1-3-5-6(7)8-4-2/h4H2,1-2H3
InChI key
FCJJZKCJURDYNF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Ethyl 2-butynoate, a 2-alkynoate is a methanogenesis inhibitor. It is an electron deficient internal alkyne that has been reported to undergo codimerization with alkenes to form 1,3-dienes catalyzed by rhodium(I)/H8-BINAP complex. The annulation of thioamides with ethyl 2-butynoate catalyzed by tri-n-butylphosphine to form thiazolines has been investigated.
Application
Ethyl 2-butynoate was used in the study of its effect on in vitro degradation and microbial biomass production.
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
- 3-ethyl, 1-methyl 1-(2-nitrophenyl)-cyclopent-3-ene-1,3-dicarboxylate
- tricyclic aziridine derivatives
- alkenylsilanols
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
143.6 °F - closed cup
Flash Point(C)
62 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of 2-azaspiro [4.4] nonan-1-ones via phosphine-catalysed [3+ 2]-cycloadditions.
Yong SR, et al.
Tetrahedron, 61(34), 8120-8129 (2005)
Bing Liu et al.
The Journal of organic chemistry, 67(13), 4595-4598 (2002-06-22)
The annulation of thioamides with 2-alkynoates and 2,3-dienoates under the catalysis of tri-n-butylphosphine was described. The annulation reaction provided a new entry to thiazolines, particularly those with 2-aryl substituents.
Yu Shibata et al.
Organic letters, 10(13), 2829-2831 (2008-06-12)
A cationic rhodium(I)/H(8)-BINAP complex catalyzes codimerization of alkenes bearing no alpha-hydrogen and electron-deficient internal alkynes, leading to 1,3-dienes in good yields with moderate to excellent regio- and stereoselectivity. The same complex also catalyzes codimerization of an acrylate and phenyl-substituted electron-rich
Dichloro [TADDOLato (2-)-O,O'] titanium/Dichlorobis [1-methylethoxy] titanium-Mediated, Highly Diastereo-and Enantioselective Additions of Silyl Enol Ethers to Nitro Olefins and [3+ 2] Cycloadditions of Primary Adducts to Acetylenes.
Seebach D, et al.
Helvetica Chimica Acta, 82(11), 1829-1842 (1999)
Efficient and stereoselective cross-coupling with highly substituted alkenylsilanols.
Denmark SE and Pan W.
Journal of Organometallic Chemistry, 653(1), 98-104 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)







