407607
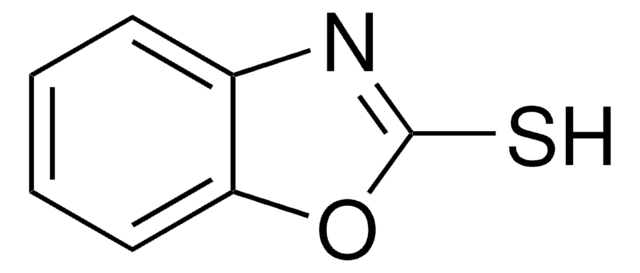
2-Hydroxybenzothiazole
98%
Synonym(s):
2(3H)-Benzothiazolone
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H5NOS
CAS Number:
Molecular Weight:
151.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
137-140 °C (lit.)
SMILES string
Oc1nc2ccccc2s1
InChI
1S/C7H5NOS/c9-7-8-5-3-1-2-4-6(5)10-7/h1-4H,(H,8,9)
InChI key
YEDUAINPPJYDJZ-UHFFFAOYSA-N
General description
2-Hydroxybenzothiazole (2-OHBT) is a 2-substituted benzothiazole. It is a tautomer of 2-benzothiazolinone. Its enthalpy of formation in the gas phase has been determined using high-level ab initio molecular orbital calculations at the G3(MP2)//B3LYP level of theory. Polarized IR spectra of the hydrogen-bonded molecular crystals of 2-OHBT have been studied. Oxidation of 2-OHBT using H2O2/UV and iron(III) photoassisted Fenton techniques have been reported. 2-OHBT is released into wastewaters during the industrial production of 2-mercaptobenzothiazole, a rubber vulcanization accelerator. The anodic oxidation of 2-OHBT on copper, iron and platinum in alcohol and alcohol-water solutions by cyclic polarization and chronoamperometry has been reported.
Application
2-Hydroxybenzothiazole may be employed as carbon, nitrogen and energy supplement in the bacterial cultures. It may be used in the preparation of insulating thin polymer (<0.1μm), via electropolymerization.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Georgia Gatidou et al.
Chemosphere, 241, 125071-125071 (2019-11-07)
The ready biodegradability of twenty food additives, belonging to the classes of artificial sweeteners, natural sweeteners, preservatives and colorings, was investigated using activated sludge as inoculum and OECD 301F respirometric test. According to the results, saccharin, aspartame, sodium cyclamate, xylitol
Influence of molecular electronic properties on the IR spectra of dimeric hydrogen bond systems: polarized spectra of 2-hydroxybenzothiazole and 2-mercaptobenzothiazole crystals.
Flakus HT, et al.
Journal of Molecular Structure, 604(1), 29-44 (2002)
Electropolymerization of 2-hydroxybenzothiazole (2-OHBT) in water-methanol media: electrochemical behaviour in NaCl (3%) solution.
Perrin FX, et al.
J. Appl. Electrochem., 27(7), 821-830 (1997)
Microbial transformations of 2-substituted benzothiazoles.
De Wever HPB and Verachtert H.
Applied Microbiology and Biotechnology, 57(5-6), 620-625 (2001)
Isolation and characterization of Rhodococcus rhodochrous for the degradation of the wastewater component 2-hydroxybenzothiazole.
De Wever H, et al.
Applied Microbiology and Biotechnology, 47(4), 458-461 (1997)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service