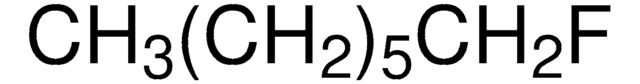All Photos(1)
About This Item
Linear Formula:
CH3(CH2)4F
CAS Number:
Molecular Weight:
90.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.36 (lit.)
bp
62-63 °C (lit.)
density
0.789 g/mL at 25 °C (lit.)
SMILES string
CCCCCF
InChI
1S/C5H11F/c1-2-3-4-5-6/h2-5H2,1H3
InChI key
OEPRBXUJOQLYID-UHFFFAOYSA-N
General description
Dual luminescence of 4-N,N-dimethylaminobenzonitrile has been observed in 1-fluoropentane.
Application
1-Fluoropentane has been used in the synthesis of 6-F-B10H13 and 6-Cl- B10H13 (halodecaboranes).
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-9.4 °F - closed cup
Flash Point(C)
-23 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The role of the solvent in the dual luminescence of 4-N, N-dimethylaminobenzonitrile.
Suppan P.
Chemical Physics Letters, 128(2), 160-161 (1986)
C F Nhachi et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 26(8), 705-713 (1988-08-01)
Phenobarbitone pretreatment potentiated hepatocyte lesions in male rats 24 hr after treatment with 1-fluoropentane (3.5 mg/kg body weight) and 1-fluorohexane (0.17 mg/kg body weight). Serum levels of the enzymes ornithine carbamyltransferase, glutamic-pyruvic transaminase and gamma-glutamyltranspeptidase were significantly elevated by the
William C Ewing et al.
Inorganic chemistry, 47(19), 8580-8582 (2008-08-30)
The high-yield syntheses of 6-X-B 10H 13 [X = Cl (88%), Br (96%), I (84%)] resulted from the cage-opening reactions of the (NH 4 (+)) 2B 10H 10 (2-) salt with ionic-liquid-based superacidic hydrogen halides, while both the previously unknown
K R Harikumar et al.
Nature chemistry, 1(9), 716-721 (2010-12-03)
The controlled imprinting of surfaces with specified patterns is important in the development of nanoscale devices. Previously, such patterns were created using self-assembled physisorbed adsorbate molecules that can be stabilized on the surface by subsequent chemical bonding. Here we show
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service