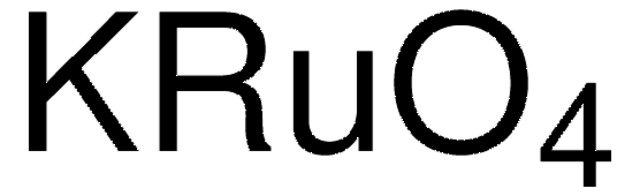209317
Copper chromite
Synonym(s):
Copperchromium oxide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
2CuO · Cr2O3
CAS Number:
Molecular Weight:
311.08
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
EC Index Number:
2346346
NACRES:
NA.23
Recommended Products
form
powder
Quality Level
application(s)
battery manufacturing
SMILES string
O=[Cu].O=[Cr]O[Cr]=O
InChI
1S/2Cr.Cu.4O
InChI key
FULFYAFFAGNFJM-UHFFFAOYSA-N
Related Categories
General description
Copper chromite is a black powder and inorganic pigment thatadopts a spinel crystal structure. It can be made by the high-temperaturecalcination of copper(II) oxide and chromium(III) oxide or the thermaldecomposition of copper chromate, which evolves oxygen at higher temperaturesand decomposes to copper chromite.
Application
Copper chromite is primarily used as a catalyst for hydrogenation reactions because of its ability to hydrogenate functional groups in aliphatic and aromatic compounds selectively. Industrially, copper chromite is used to reduce furfural to furfuryl alcohol and butyraldehyde to 1-butanol,partially reduce conjugated dienes to monoenes, and selectively reduce carbonyl groups in vegetable oils. This CuCr catalyst is studied for a variety of catalytic applications including converting the hydrogenolysis of cellulose.It is also used as a catalyst for combustion to help control burn rate and as alight-absorbing pigment. Copper chromite/graphene oxide nanocomposite canbe used in energy storage applications.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Ox. Sol. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Deactivation mechanistic studies of copper chromite catalyst for selective hydrogenation of 2-furfuraldehyde.
Liu D, et al.
J. Catal., 299, 336-345 (2013)
Pravin P Upare et al.
ChemSusChem, 13(18), 5050-5057 (2020-07-15)
We report for the first time the selective production of mannitol, a low-calorie sweetener and an important pharmaceutical ingredient, from fructose using Cu-SiO2 nanocomposite as catalyst and 1-butanol as solvent. When compared with water and ethanol, a lower fructose solubility
Conversion of highly concentrated cellulose to 1,2-propanediol and ethylene glycol over highly efficient CuCr catalysts.
Xaio Z, et al.
Green Chemistry, 15, 891-895 (2013)
Maura Koehle et al.
ChemSusChem, 10(1), 91-98 (2016-12-13)
A four-step catalytic process was developed to produce p-methylstyrene from methylfuran, a biomass-derived species. First, methylfuran was acylated over zeolite H-Beta with acetic anhydride. Second, the acetyl group was reduced to an ethyl group with hydrogen over copper chromite. Third
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service