All Photos(1)
About This Item
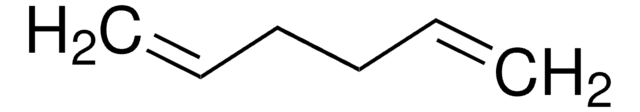
Linear Formula:
HC≡C(CH2)4C≡CH
CAS Number:
Molecular Weight:
106.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.446 (lit.)
bp
135-136 °C (lit.)
density
0.8 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C#CCCCCC#C
InChI
1S/C8H10/c1-3-5-7-8-6-4-2/h1-2H,5-8H2
InChI key
DSOJWVLXZNRKCS-UHFFFAOYSA-N
Related Categories
General description
1,7-Octadiyne is a terminal bis-alkyne and participates in the formation of uranium(IV) vinyl complexes. It reacts with frustrated Lewis pair P(o-tolyl)3/B(C6F5)3 by acetylene C-C coupling to yield the zwitterionic product. It undergoes the reductive cyclization during reaction with Re2Cl4(μ-dppm)2 (dppm = Ph2PCH2PPh2) and affords quadruply bonded dirhenium(III) complex Re2Cl3(μ,η2-C8H7)(μ-dppm)2.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
73.4 °F - closed cup
Flash Point(C)
23 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Novel Example of the Reductive Cyclization of a Diyne at a Re-Re Triple Bond: The Reaction of Re2Cl4 (?-dppm) 2 with 1, 7-Octadiyne.
Ganesan M, et al.
Organometallics, 22(4), 870-872 (2003)
Christina A Braun et al.
Dalton transactions (Cambridge, England : 2003), 48(27), 10210-10219 (2019-06-14)
A new di(isopropoxy)boryl -B(OiPr)2 tellurophene precursor is described, from which several previously inaccessible phosphorescent borylated tellurophenes are formed via exchange of the -OiPr groups. One such tellurophene Mes(iPrO)B-Te-6-B(OiPr)Mes, bearing a sterically encumbered mesityl (Mes) substituent at each boron center, exhibits
Boris Kosog et al.
Journal of the American Chemical Society, 134(30), 12792-12797 (2012-06-29)
The previously reported uranium(III) complex [(((Ad)ArO)(3)N)U(III)(DME)] (1; Ad = adamantane, DME = 1,2-dimethoxyethane) reacts with the terminal bis-alkynes 1,7-octadiyne or 1,6-heptadiyne in C-C-coupling reactions to form the uranium(IV) vinyl complexes [{(((Ad)ArO)(3)N)U(IV)}(2)(μ-η(2):η(1)-1,2-(CH)(2)-cyclohexane)] (2) and [{(((Ad)ArO)(3)N)U(IV)}(2)(μ-η(2):η(2)-1,2-(CH)(2)-cyclopentane)] (3). With the monoalkynes 1-hexyne or
Chao Chen et al.
Chemical communications (Cambridge, England), 46(20), 3580-3582 (2010-04-09)
The frustrated Lewis pair P(o-tolyl)(3)/B(C(6)F(5))(3) reacts with 1,7-octadiyne by acetylene C-C coupling to yield the zwitterionic product 2a. In contrast, the P(o-tolyl)(3)/B(C(6)F(5))(3) Lewis pair reacts with 1,6-heptadiyne by a sequence involving 1,1-carboboration of a terminal acetylene to eventually yield the
Alla V Lipeeva et al.
Molecules (Basel, Switzerland), 24(11) (2019-06-15)
Synthesis of 1,2,3-triazole-substituted coumarins and also 1,2,3-triazolyl or 1,2,3-triazolylalk-1-inyl-linked coumarin-2,3-furocoumarin hybrids was performed by employing the cross-coupling and copper catalyzed azide-alkyne cycloaddition reaction approaches. The synthesized compounds were evaluated for their in vitro antibacterial activity against Staphylococcusaureus, Bacilliussubtilis, Actinomycesviscosus and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service