114286
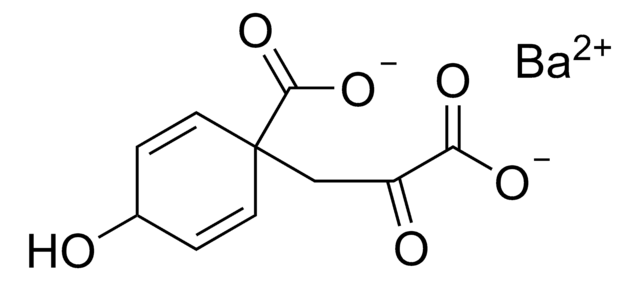
4-Hydroxyphenylpyruvic acid
98%
Synonym(s):
3-(4-Hydroxyphenyl)-2-oxopropanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H4CH2COCO2H
CAS Number:
Molecular Weight:
180.16
Beilstein:
2691632
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
219-220 °C (dec.) (lit.)
solubility
ethanol: soluble 50 mg/mL
functional group
carboxylic acid
ketone
SMILES string
OC(=O)C(=O)Cc1ccc(O)cc1
InChI
1S/C9H8O4/c10-7-3-1-6(2-4-7)5-8(11)9(12)13/h1-4,10H,5H2,(H,12,13)
InChI key
KKADPXVIOXHVKN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-hydroxyphenylpyruvic acid can be determined in pork meat and Iberian ham samples by a sensitive method of multiple reaction monitoring (MRM) by mass spectrometry.
Application
<ul>
<li><strong>Identification of serum biomarkers of ischemic stroke:</strong>4-Hydroxyphenylpyruvic acid is used as a potential diagnostic biomarker in the study to distinguish hypertensive ischemic stroke (IS) patients from both healthy individuals and those with hypertension (Zhao et al., 2023).</li>
</ul>
<li><strong>Identification of serum biomarkers of ischemic stroke:</strong>4-Hydroxyphenylpyruvic acid is used as a potential diagnostic biomarker in the study to distinguish hypertensive ischemic stroke (IS) patients from both healthy individuals and those with hypertension (Zhao et al., 2023).</li>
</ul>
Preparation Note
50 gm of 4-Hydroxyphenylpyruvic acid dissolves in 1 mL of ethanol to yield a clear, light yellow solution.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Francisco J Hidalgo et al.
Food chemistry, 140(1-2), 183-188 (2013-04-13)
An analytical method which offers accurate determination and identification of eight α-keto acids (α-ketoglutaric acid, pyruvic acid, 4-hydroxyphenylpyruvic acid, 3-methyl-2-oxobutyric acid, α-keto-γ-methylthiobutyric acid, 4-methyl-2-oxovaleric acid, 3-methyl-2-oxovaleric acid, and phenylpyruvic acid) in pork meat and Iberian ham samples is reported. The
Tomasz Borowski et al.
Biochemistry, 43(38), 12331-12342 (2004-09-24)
Density functional calculations using the B3LYP functional has been used to study the reaction mechanism of 4-hydroxyphenylpyruvate dioxygenase. The first part of the catalytic reaction, dioxygen activation, is found to have the same mechanism as in alpha-ketoglutarate-dependent enzymes; the ternary
Nick J P Wierckx et al.
Journal of bacteriology, 190(8), 2822-2830 (2007-11-13)
The unknown genetic basis for improved phenol production by a recombinant Pseudomonas putida S12 derivative bearing the tpl (tyrosine-phenol lyase) gene was investigated via comparative transcriptomics, nucleotide sequence analysis, and targeted gene disruption. We show upregulation of tyrosine biosynthetic genes
Ute Metzger et al.
Journal of molecular biology, 404(4), 611-626 (2010-10-16)
CloQ is an aromatic prenyltransferase from the clorobiocin biosynthetic pathway of Streptomyces roseochromogenes var. oscitans. It is involved in the synthesis of the prenylated hydroxybenzoate moiety of the antibiotic, specifically catalyzing the attachment of a dimethylallyl moiety to 4-hydroxyphenylpyruvate. Herein
Dingding Shao et al.
Acta tropica, 106(1), 9-15 (2008-02-12)
Macrophage migration inhibitory factor homologues have been identified from several genera of parasites, including Plasmodium, and have shown some functional similarities to the host molecule. It was hypothesized that MIF molecules can act as a regulator in host-parasite interaction in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service