A0980
AM281
≥98% (HPLC)
Synonyme(s) :
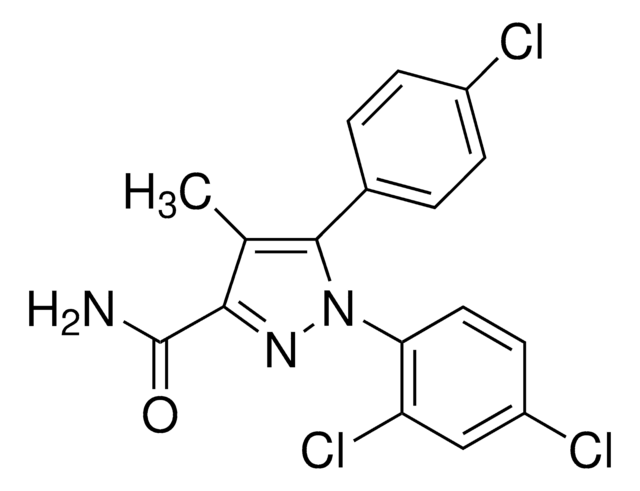
1-(2,4-Dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide, AM 281
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥98% (HPLC)
Forme
powder
Conditions de stockage
desiccated
Couleur
white
Solubilité
DMSO: >6 mg/mL
H2O: insoluble
Température de stockage
2-8°C
Chaîne SMILES
Cc1c(nn(-c2ccc(Cl)cc2Cl)c1-c3ccc(I)cc3)C(=O)NN4CCOCC4
InChI
1S/C21H19Cl2IN4O2/c1-13-19(21(29)26-27-8-10-30-11-9-27)25-28(18-7-4-15(22)12-17(18)23)20(13)14-2-5-16(24)6-3-14/h2-7,12H,8-11H2,1H3,(H,26,29)
Clé InChI
AJFFBPZYXRNAIC-UHFFFAOYSA-N
Informations sur le gène
rat ... Cnr1(25248)
Application
- to study its effects on memory deficit following naloxone-precipitated morphine withdrawal in mice
- to study the role of CB1 receptor system in modulating acetaldehyde-induced effects in rats during the extinction-, relapse-, and conflict-experiments
- to study its effect on scopolamine-induced memory deficit using object recognition paradigm
- to block synthetic cannabinoid (HU210)-induced analgesia in the ventrolateral orbital cortex (VLO) to evaluate the effect of CB1 receptors on the VLO modulation of pain
Actions biochimiques/physiologiques
Caractéristiques et avantages
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 2 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique