SMB01324
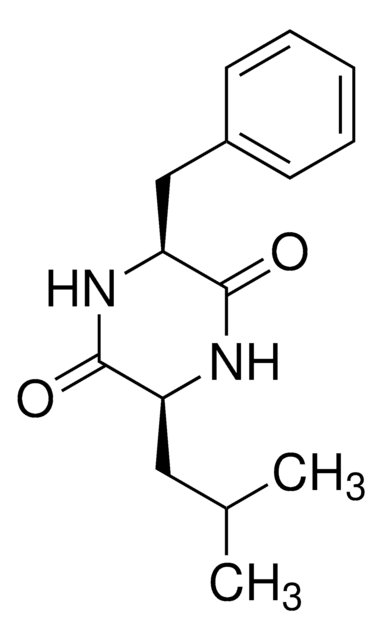
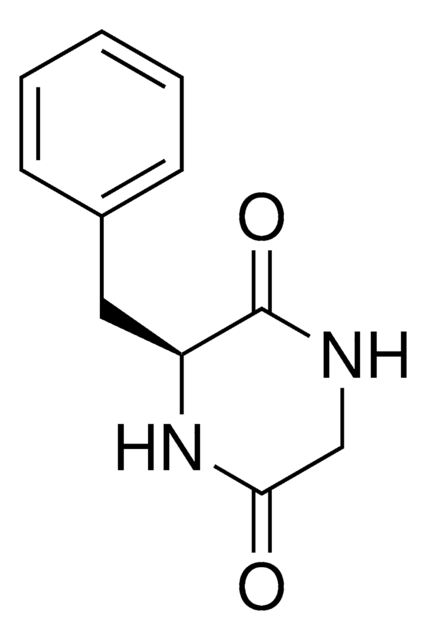
Cyclo(His-Pro)
Synonyme(s) :
Cyclo(L-histidyl-L-proline), Cyclo(histidyl-proline), Cyclo(prolylhistidine), Histidylproline diketopiperazine, Histidylproline dioxopiperazine, CHP
About This Item
Produits recommandés
Pureté
98% (HPLC)
Niveau de qualité
Forme
solid
Couleur
white to off-white
Pf
162—164 °C
Conditions d'expédition
2-8°C
Température de stockage
−20°C
Chaîne SMILES
O=C1N[C@@H](Cc2cnc[nH]2)C(=O)N3CCC[C@@H]13
InChI
1S/C11H14N4O2/c16-10-9-2-1-3-15(9)11(17)8(14-10)4-7-5-12-6-13-7/h5-6,8-9H,1-4H2,(H,12,13)(H,14,16)/t8-,9-/m0/s1
Clé InChI
NAKUGCPAQTUSBE-IUCAKERBSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Caractéristiques et avantages
- Can be used in Metabolomics and Biochemical research
- High-quality compound suitable for multiple research applications
Autres remarques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Choose from one of the most recent versions:
Certificats d'analyse (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique