SMB00930
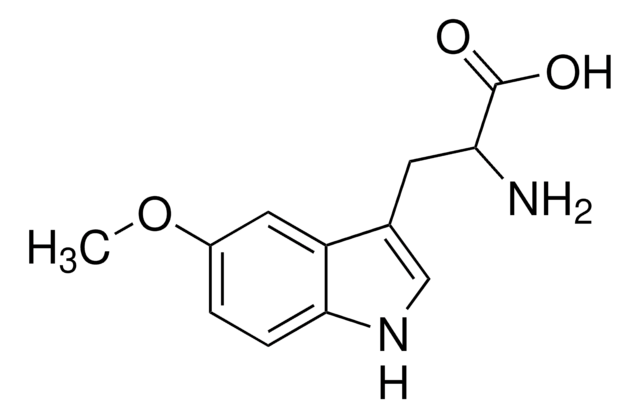
5-Methoxy-L-tryptophan
98-102%
Synonyme(s) :
5-Methoxy-L-tryptophan, L-2-Amino-3-(5-methoxyindolyl)propionic acid
About This Item
Produits recommandés
Niveau de qualité
Pureté
98-102%
Forme
solid
Activité optique
[α]20/D -30 to -28° in water
Couleur
off-white to light yellow
Température de stockage
2-8°C
InChI
1S/C12H14N2O3/c1-17-8-2-3-11-9(5-8)7(6-14-11)4-10(13)12(15)16/h2-3,5-6,10,14H,4,13H2,1H3,(H,15,16)/t10-/m0/s1
Clé InChI
KVNPSKDDJARYKK-JTQLQIEISA-N
Description générale
Application
Caractéristiques et avantages
- Can be used in Metabolomics and Biochemical research
- High-quality compound suitable for multiple research applications
Autres remarques
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Oral
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Choose from one of the most recent versions:
Certificats d'analyse (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique