36849
Fenoxaprop
PESTANAL®, analytical standard
Synonyme(s) :
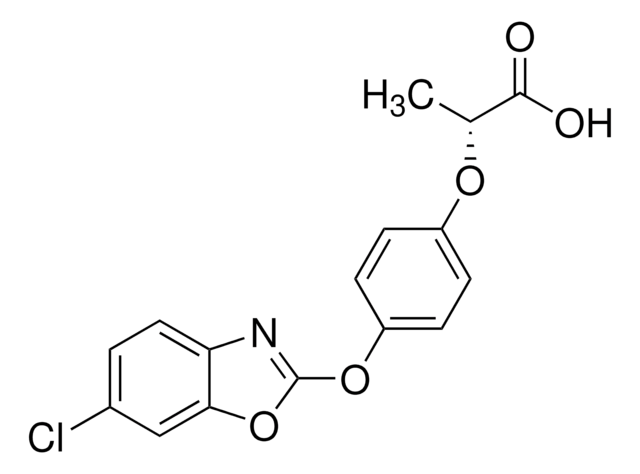
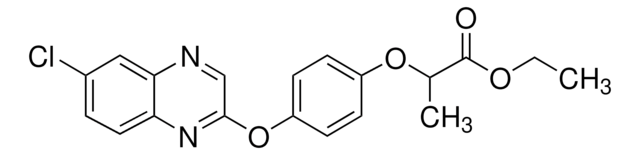
2-{4-[(6-Chlorobenzoxazol-2-yl)oxy]phenoxy}propionic acid
About This Item
Produits recommandés
Qualité
analytical standard
Niveau de qualité
Gamme de produits
PESTANAL®
Durée de conservation
limited shelf life, expiry date on the label
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Application(s)
agriculture
environmental
Format
neat
Température de stockage
2-8°C
Chaîne SMILES
CC(Oc1ccc(Oc2nc3ccc(Cl)cc3o2)cc1)C(O)=O
InChI
1S/C16H12ClNO5/c1-9(15(19)20)21-11-3-5-12(6-4-11)22-16-18-13-7-2-10(17)8-14(13)23-16/h2-9H,1H3,(H,19,20)
Clé InChI
MPPOHAUSNPTFAJ-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
According to Commission Regulation (1107/2009), fenoxaprop is not approved for use as a plant protection product in the European Union. However, a default MRL of 0.01 mg/kg is set according to Art 18(1)(b) Reg 396 / 2005.
Application
- Develop an HPLC method for the determination of fenoxaprop-ethyl and fenoxaprop residues in four soil types using two extraction procedures
- Validate the resistance of fenoxaprop in wild oats in Turkey and investigate cross and multiple resistance patterns of fenoxaprop-resistant wild oat populations
- Investigate the degradation of fenoxaprop-ethyl and fenoxaprop in three soils were under native and sterilized conditions using enantioselective high-performance liquid chromatography (HPLC)
- Determine the effect of soil moisture, temperature, and light intensity on the spray deposition of fenoxaprop and imazamethabenz applied to wild oat plants
- Investigate and quantify the resistance of Japanese foxtail (Alopecurus japonicus) to fenoxaprop and pinoxaden in China and elucidate the basis of resistance to these herbicides
Informations légales
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Acute 1 - Aquatic Chronic 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Filtres actifs
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique