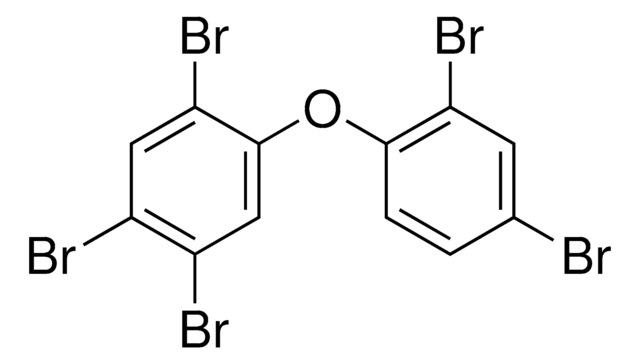
33684
BDE Nr. 154 solution
50 μg/mL in isooctane, analytical standard
Synonyme(s) :
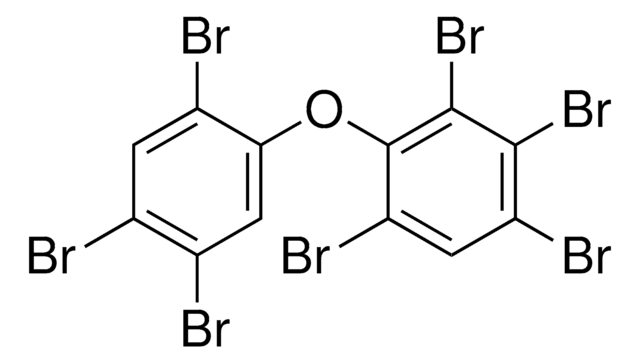
2,2′,4,4′,5,6′-Hexabromodiphenyl ether solution, PBDE 154
About This Item
Produits recommandés
Qualité
analytical standard
Durée de conservation
limited shelf life, expiry date on the label
Concentration
50 μg/mL in isooctane
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Application(s)
environmental
Format
single component solution
Température de stockage
2-8°C
Chaîne SMILES
Brc1cc(Br)c(Oc2cc(Br)c(Br)cc2Br)c(Br)c1
InChI
1S/C12H4Br6O/c13-5-1-9(17)12(10(18)2-5)19-11-4-7(15)6(14)3-8(11)16/h1-4H
Clé InChI
VHNPZYZQKWIWOD-UHFFFAOYSA-N
Description générale
Application
- House dust samples using isotope dilution method combined with liquid chromatography coupled to negative ionization atmospheric pressure photoionization tandem mass spectrometry (LC-NI-APPI-MS/MS).
- Adipose tissue samples using gas chromatography coupled to ion-trap mass spectrometry (GC-ITMS).
Autres remarques
The standard should be transferred to a clean and appropriate vial or flask using clean pipettes or micro pipettes. The vial should be immediately capped to avoid any loss or evaporation of the solvent.
After opening the ampoule, the standard should not be stored or kept in the ampoule. To preserve the integrity of the product, the standard should be transferred to an appropriate vial that must be capped and stored according to the recommendation on the Certificate of Analysis.
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Central nervous system
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
10.4 °F
Point d'éclair (°C)
-12 °C
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique