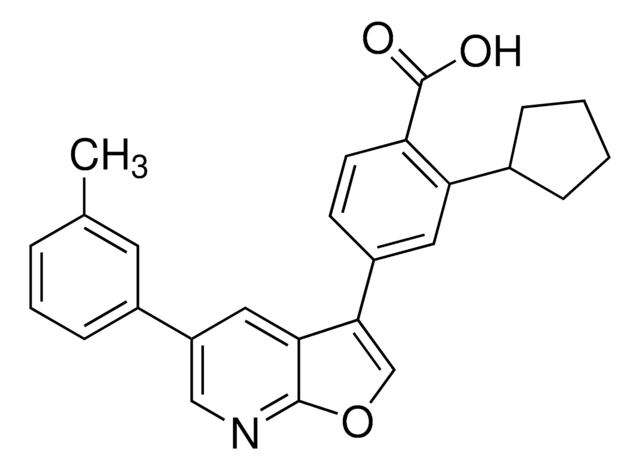
CC1047
MMP-13, human, proform, his-tagged, E. coli recombinant
Synonyme(s) :
Procollagenase 3
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Code UNSPSC :
12352204
eCl@ss :
32160405
Nomenclature NACRES :
NA.77
Produits recommandés
Source biologique
human
Niveau de qualité
Pureté
>95% (total protein)
Forme
liquid
Activité spécifique
250-300 mU/mg
Fabricant/nom de marque
Chemicon®
Concentration
0.2 μg/μL
Numéro d'accès NCBI
Numéro d'accès UniProt
Conditions d'expédition
dry ice
Informations sur le gène
human ... MMP13(4322)
Description générale
CC1047 is a recombinant 452 amino acid polypeptide corresponding to human Pro-MMP-13, with an additional C-terminal His-tag with the sequence GVTHHHHHH expressed in E. coli and purified from periplasm. The calculated Mr of the recombinant protein is 51.681 kDa. Upon activation with APMA activated MMP-13 is formed.
Matrix metalloproteinases (MMPs) are Zn2+- and Ca2+-dependent endopeptidases which function in the turnover of extracellular matrix components [Matrisian, 1992]. Main subfamilies of MMP are collagenases, gelatinases, stromelysins and membrane-type matrix metalloproteinases [Nagase, 1997]. Three homologous collagenases have been identified in human tissues: Interstitial collagenase, neutrophil collagenase and collagenase-3. These three enzymes cleave fibrillar collagens at a single site, generating fragments of approximately ¾ and ¼ the size of the original molecules.
ProMMP-13 [Procollagenase-3] consists of 452 amino acids with a calculated Mr of 52.520 [Freije et al., 1994]. Due to N-linked glycosylation, the actual Mr is about 60000 Da [Knauper et al., 1996]. Within the protein the following domains and sequence regions can be distinguished [Freije et al., 1994; Knauper et al., 1996]: An N-terminal propeptide, which confers latency to the proenzyme, a Ca2+ and Zn2+-ion binding catalytic domain, a hinge region, and a C-terminal hemopexin-like domain. Latent procollagenase-3 can be activated by proteases such as stromelysin [ Knauper et al., 1996], gelatinase A, MT1-MMP and plasmin [ Knauper et al., 1996] or incubation with APMA Knauper et al., 1996]. The Mr of active collagenase-3 which begins with the N-terminal sequence YNVFPRTL is 48,000 Da.
Collagenase-3 hydrolyzes type II collagen 5- to 6- times faster than type I and type III collagens. The enzyme also exhibits high activity towards gelatin and it degrades SERPINS as a1-antichymotrypsin and plasminogen activator inhibitor-2 [Knauper et al., 1996]. Collagenase-3 is inhibited in a 1:1 stoichiometric fashion by TIMP-1, TIMP-2 and TIMP-3.
Collagenase-3 is expressed during fetal bone development [Stahlebackdahl, 1997]. In adult human tissues collagenase-3 has been detected only in pathological conditions: in malignant tumors [Freije et al., 1994], in chronic ulcers [Valaamo et al., 1997], in arthritic cartilage [Mitchell et al., 1996] and synovium [Wernicke et al., 1996].
ProMMP-13 [Procollagenase-3] consists of 452 amino acids with a calculated Mr of 52.520 [Freije et al., 1994]. Due to N-linked glycosylation, the actual Mr is about 60000 Da [Knauper et al., 1996]. Within the protein the following domains and sequence regions can be distinguished [Freije et al., 1994; Knauper et al., 1996]: An N-terminal propeptide, which confers latency to the proenzyme, a Ca2+ and Zn2+-ion binding catalytic domain, a hinge region, and a C-terminal hemopexin-like domain. Latent procollagenase-3 can be activated by proteases such as stromelysin [ Knauper et al., 1996], gelatinase A, MT1-MMP and plasmin [ Knauper et al., 1996] or incubation with APMA Knauper et al., 1996]. The Mr of active collagenase-3 which begins with the N-terminal sequence YNVFPRTL is 48,000 Da.
Collagenase-3 hydrolyzes type II collagen 5- to 6- times faster than type I and type III collagens. The enzyme also exhibits high activity towards gelatin and it degrades SERPINS as a1-antichymotrypsin and plasminogen activator inhibitor-2 [Knauper et al., 1996]. Collagenase-3 is inhibited in a 1:1 stoichiometric fashion by TIMP-1, TIMP-2 and TIMP-3.
Collagenase-3 is expressed during fetal bone development [Stahlebackdahl, 1997]. In adult human tissues collagenase-3 has been detected only in pathological conditions: in malignant tumors [Freije et al., 1994], in chronic ulcers [Valaamo et al., 1997], in arthritic cartilage [Mitchell et al., 1996] and synovium [Wernicke et al., 1996].
Application
ACTIVATION:
An aliquot of 19.5 μL procollagenase-3 is mixed with 0.5 μL APMA solution (40 mM p-aminophenyl mercuric acetate in DMSO) and the mixture is incubated for 30 minutes at 37°C. The mixture may be stored on ice until use for activity assays.
INHIBITORS:
MMP-13 is inhibited by TIMPs and by chelators of divalent cations such as EDTA or o-phenanthroline.
An aliquot of 19.5 μL procollagenase-3 is mixed with 0.5 μL APMA solution (40 mM p-aminophenyl mercuric acetate in DMSO) and the mixture is incubated for 30 minutes at 37°C. The mixture may be stored on ice until use for activity assays.
INHIBITORS:
MMP-13 is inhibited by TIMPs and by chelators of divalent cations such as EDTA or o-phenanthroline.
Définition de l'unité
Specific Activity: where 1 U is the activity that hydrolyzes 1 μmol peptide (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-Dpa-Ala-Arg) within 1 minute (Knight, 1992).
Forme physique
Provided as a liquid in 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM CaCl2, 0.05% Brij-35.
Stockage et stabilité
Maintain frozen at -70°C in undiluted aliquots. The enzyme may be stored at -20°C for several weeks without significant loss of activity. Repeated freezing and thawing should be avoided.
Remarque sur l'analyse
Pro-MMP-13 appears as a major band at about 60 kDa in SDS-PAGE
Informations légales
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Clause de non-responsabilité
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds.
Vaalamo, M, et al.
The Journal of Investigative Dermatology, 109, 96-101 (1997)
P G Mitchell et al.
The Journal of clinical investigation, 97(3), 761-768 (1996-02-01)
Proteolysis of triple-helical collagen is an important step in the progression toward irreversible tissue damage in osteoarthritis. Earlier work on the expression of enzymes in cartilage suggested that collagenase-1 (MMP-1) contributes to the process. Degenerate reverse transcription polymerase chain reaction
Activation mechanisms of matrix metalloproteinases.
Nagase, H
Biological Chemistry, 378, 151-160 (1997)
Characterization of an exosite binding inhibitor of matrix metalloproteinase 13.
Lata T Gooljarsingh,Ami Lakdawala,Frank Coppo,Lusong Luo,Gregg B Fields,Peter J Tummino et al.
Protein Science null
Cloning of collagenase 3 from the synovial membrane and its expression in rheumatoid arthritis and osteoarthritis.
Wernicke, D, et al.
The Journal of Rheumatology, 23, 590-595 (1996)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique