900555
(N-Isocyanoimino)triphenylphosphorane
95%
Synonyme(s) :
Pinc
About This Item
Produits recommandés
Niveau de qualité
Pureté
95%
Forme
solid
Application(s)
peptide synthesis
InChI
1S/C19H15N2P/c1-20-21-22(17-11-5-2-6-12-17,18-13-7-3-8-14-18)19-15-9-4-10-16-19/h2-16H
Clé InChI
NIDTXBFHPXMXTR-UHFFFAOYSA-N
Description générale

Application
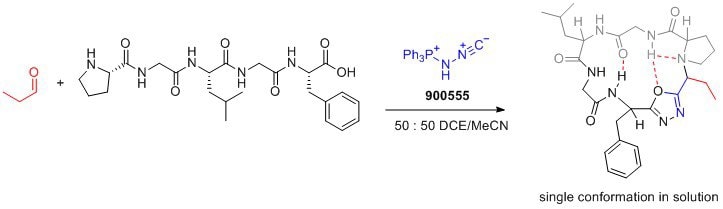
Caractéristiques et avantages
- Bench-stable solid: Pinc is a stable reagent that can be easily handled and stored.
- Facilitates cyclization and incorporation of conformational control element: Pinc enables the formation of peptide macrocycles with a desired conformation, leading to improved drug properties.
- Amphoteric properties: Pinc′s amphoteric nature allows for the design of new multicomponent reactions, expanding its synthetic capabilities.
- Desired drug properties: The resulting peptide macrocycles exhibit enhanced membrane permeability, lipophilicity, and aqueous solubility, making them desirable for drug development.
- Versatile transformations: Pinc′s ability to form oxadiazoles opens up opportunities for the development of novel synthetic transformations.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Isocyanides are widely used reagents in organic synthesis, with applications ranging from materials science to drug discovery.
Contenu apparenté
The Yudin laboratory is known for the development of amphoteric molecules and their application in synthesis. The corresponding reagents possess nucleophilic and electrophilic functional groups that do not prematurely react with each other.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique
![Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde 96%](/deepweb/assets/sigmaaldrich/product/structures/137/771/57dfbc98-f02d-4773-bc11-3e8b861ad74b/640/57dfbc98-f02d-4773-bc11-3e8b861ad74b.png)