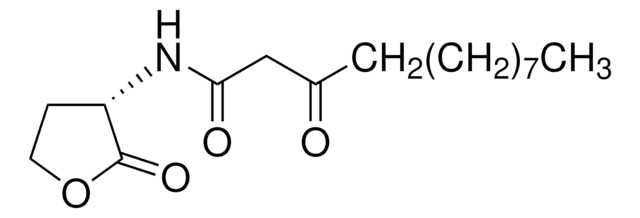
804223
3-oxo-C12-aniline
Synonyme(s) :
3-oxo-N-phenyl-Dodecanamide, LasR inhibitor, non-hydrolysable head group
About This Item
Produits recommandés
Forme
solid
Température de stockage
−20°C
Chaîne SMILES
CCCCCCCCCC(CC(NC1=CC=CC=C1)=O)=O
InChI
1S/C18H27NO2/c1-2-3-4-5-6-7-11-14-17(20)15-18(21)19-16-12-9-8-10-13-16/h8-10,12-13H,2-7,11,14-15H2,1H3,(H,19,21)
Clé InChI
GYOZLHQAMICVMN-UHFFFAOYSA-N
Application
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Contenu apparenté
Our laboratory pursues research at the chemistry-microbiology interface. We are deeply interested in the mechanisms by which bacteria sense each other, their environment, and the eukaryotic hosts on which and in which they may reside. One prominent pathway that we study is called quorum sensing, which allows bacteria to assess their local population density and initiate group behaviors at high cell (or “quorate”) density. This pathway allows, for example, many pathogens to amass in large populations prior to attacking their hosts. Bacteria use chemical signals for quorum sensing, and it is the concentration of these signals in a given environment that alerts the bacteria to their current cell number. We are interested in the structures of these signals and how we can reengineer them to either ablate or amplify quorum-sensing networks. Through synthesis and systematic screening, we have identified critical structural features of these signals and non-native functionality that we can install into the signals to tune their function. Thereby, we have developed non-native molecules that strongly inhibit or activate quorum-sensing pathways and modify infection processes. These compounds represent useful tools to explore the role of quorum sensing in many biological processes. We are applying them to both study fundamental aspects of quorum sensing pathways, and examine different types of infections in animals and plants.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique