SRP4858
Heat Shock Protein 90 human
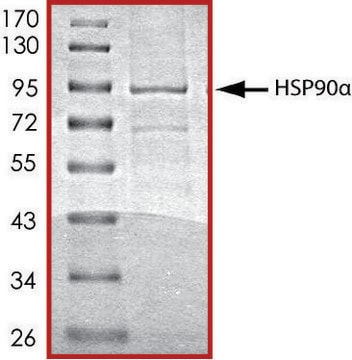
recombinant, expressed in baculovirus infected Sf9 cells, ≥99% (SDS-PAGE)
Synonym(s):
HSP86, HSPC1, HSPCA, HSPN, Hsp89, LAP2
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
human
recombinant
expressed in baculovirus infected Sf9 cells
Assay
≥99% (SDS-PAGE)
form
liquid
mol wt
~90 kDa
packaging
pkg of 25 μg
storage condition
avoid repeated freeze/thaw cycles
impurities
endotoxin, tested
NCBI accession no.
UniProt accession no.
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... HSP90B1(7184)
General description
Heat shock protein 90 (HSP90) is a molecular chaperone and one of the most abundant proteins in unstressed cells. In mammalian cells, there are two genes encoding cytosolic Hsp90 homologues, with the human Hsp90α showing 85% sequence identity to Hsp90β. The recombinant Hsp90 produced from Sf9 cells represents the full length (a.a. 1-723) of Hsp90β subunit.
Biochem/physiol Actions
Heat shock protein 90kDa beta member 1 (HSP90B1) is activated during apoptosis. It is associated with the endoplasmic reticulum membrane and undergoes specific proteolytic cleavage leading to the activation of the caspase CPP32 and initiation of DNA fragmentation. The protein protects cells against flux in Ca2+ homeostasis, which would otherwise result in cell death. It maintains the chromosomal stability in mammalian cells by associating with FAC. HSP90B1 chaperones toll-like receptors (TLRs) and thus optimizes the B cell functioning. These proteins are also the main downstream chaperones which mediate the ER unfolded protein response (UPR), which is very essential for maintaining protein homeostasis in the ER.
Physical form
Protein is provided in a solution of 40 mM HEPES/KOH, pH 7.5, containing 400 mM potassium chloride and 5% glycerol.
Preparation Note
Centrifuge the vial prior to opening. Avoid freeze-thaw cycles
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T Hoshino et al.
Blood, 91(11), 4379-4386 (1998-05-30)
The FAC protein encoded by the gene defective in Fanconi anemia (FA) complementation group C binds to at least three ubiquitous cytoplasmic proteins in vitro. We used here the complete coding sequence of FAC in a yeast two-hybrid screen to
Bei Liu et al.
Blood, 112(4), 1223-1230 (2008-05-30)
Endoplasmic reticulum (ER) unfolded protein response (UPR) plays pivotal roles in both early B-cell development and plasma cell differentiation. As a major ER chaperone to mediate the UPR and a master chaperone for Toll-like receptors (TLRs), HSP90b1 (grp94, gp96) has
R K Reddy et al.
The Journal of biological chemistry, 274(40), 28476-28483 (1999-09-25)
GRP94 is a 94-kDa chaperone glycoprotein with Ca(2+)-binding properties. We report here that during apoptosis induced by the topoisomerase II inhibitor etoposide, a fraction of GRP94 associated with the endoplasmic reticulum membrane undergoes specific proteolytic cleavage, coinciding with the activation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service