PZ0403
PF-9366
≥98% (HPLC)
Synonym(s):
7-Chloro-N,N-dimethyl-5-phenyl-[1,2,4]triazolo[4,3-a]quinoline-1-ethanamine, PF 9366, PF9366
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H19ClN4
CAS Number:
Molecular Weight:
350.84
MDL number:
UNSPSC Code:
51111800
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 2 mg/mL, clear
storage temp.
room temp
SMILES string
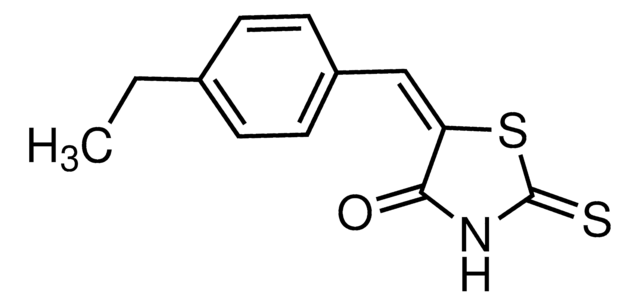
ClC(C=C1)=CC(C(C2=CC=CC=C2)=C3)=C1N4C3=NN=C4CCN(C)C
Biochem/physiol Actions
PF-9366 is a methionine adenosyltransferase 2A allosteric inhibitor (human Mat2A IC50/Kd = 420 nM/170 nM) whose binding site overlaps with that of the Mat2A regulator, Mat2B. PF-9366 inhibits cellular SAM production in a Mat2B-competitive manner (IC50 post 6-hr treatment = 1.2 μM wihout vs. 0.86 μM with Mat2B knockdown; H520 lung carcinoma cells) without antiproliferation efficacy in cancer cultures due to an induction of Mat2A upregulation upon allosteric inhibition. Similar to Mat2B, PF-9366 allosteric binding alters Mat2A active site, causing increased substrate affinity and decreased enzyme turnover.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Weiwei Yu et al.
Molecular cell, 75(6), 1147-1160 (2019-08-20)
Activated macrophages adapt their metabolic pathways to drive the pro-inflammatory phenotype, but little is known about the biochemical underpinnings of this process. Here, we find that lipopolysaccharide (LPS) activates the pentose phosphate pathway, the serine synthesis pathway, and one-carbon metabolism
Jiraporn Panmanee et al.
The FEBS journal, 286(11), 2135-2154 (2019-02-19)
Methylation is an underpinning process of life and provides control for biological processes such as DNA synthesis, cell growth, and apoptosis. Methionine adenosyltransferases (MAT) produce the cellular methyl donor, S-Adenosylmethionine (SAMe). Dysregulation of SAMe level is a relevant event in
Casey L Quinlan et al.
Nature chemical biology, 13(7), 785-792 (2017-05-30)
S-Adenosyl-L-methionine (SAM) is an enzyme cofactor used in methyl transfer reactions and polyamine biosynthesis. The biosynthesis of SAM from ATP and L-methionine is performed by the methionine adenosyltransferase enzyme family (Mat; EC 2.5.1.6). Human methionine adenosyltransferase 2A (Mat2A), the extrahepatic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service